Page 121 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 121
100
CHAPTER 1
Chemical Bonding
and Molecular Structure
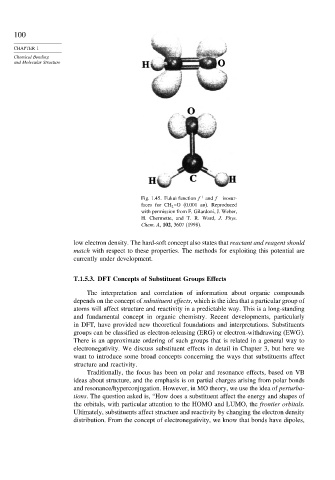
Fig. 1.45. Fukui function f + and f − isosur-
faces for CH 2 =O (0.001 au). Reproduced
with permission from F. Gilardoni, J. Weber,
H. Chermette, and T. R. Ward, J. Phys.
Chem. A, 102, 3607 (1998).
low electron density. The hard-soft concept also states that reactant and reagent should
match with respect to these properties. The methods for exploiting this potential are
currently under development.
T.1.5.3. DFT Concepts of Substituent Groups Effects
The interpretation and correlation of information about organic compounds
depends on the concept of substituent effects, which is the idea that a particular group of
atoms will affect structure and reactivity in a predictable way. This is a long-standing
and fundamental concept in organic chemistry. Recent developments, particularly
in DFT, have provided new theoretical foundations and interpretations. Substituents
groups can be classified as electron-releasing (ERG) or electron-withdrawing (EWG).
There is an approximate ordering of such groups that is related in a general way to
electronegativity. We discuss substituent effects in detail in Chapter 3, but here we
want to introduce some broad concepts concerning the ways that substituents affect
structure and reactivity.
Traditionally, the focus has been on polar and resonance effects, based on VB
ideas about structure, and the emphasis is on partial charges arising from polar bonds
and resonance/hyperconjugation. However, in MO theory, we use the idea of perturba-
tions. The question asked is, “How does a substituent affect the energy and shapes of
the orbitals, with particular attention to the HOMO and LUMO, the frontier orbitals.
Ultimately, substituents affect structure and reactivity by changing the electron density
distribution. From the concept of electronegativity, we know that bonds have dipoles,