Page 124 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 124
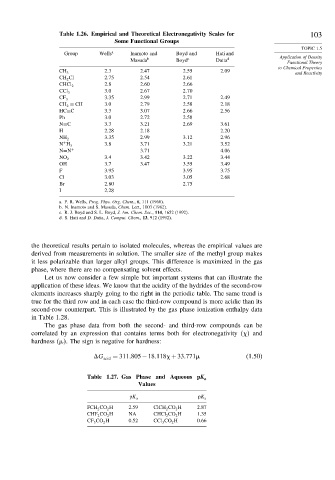
Table 1.26. Empirical and Theoretical Electronegativity Scales for 103
Some Functional Groups
TOPIC 1.5
Group Wells a Inamoto and Boyd and Hati and Application of Density
Masuda b Boyd c Datta d
Functional Theory
to Chemical Properties
2 3 2 47 2 55 2 09
CH 3
and Reactivity
CH 2 Cl 2 75 2 54 2 61
2 8 2 60 2 66
CHCl 2
3 0 2 67 2 70
CCl 3
3 35 2 99 2 71 2 49
CF 3
CH 2 = CH 3 0 2 79 2 58 2 18
HC≡C 3 3 3 07 2 66 2 56
Ph 3 0 2 72 2 58
N≡C 3 3 3 21 2 69 3 61
H 2 28 2 18 2 20
3 35 2 99 3 12 2 96
NH 2
+ 3 8 3 71 3 21 3 52
N H 3
N≡N + 3 71 4 06
3 4 3 42 3 22 3 44
NO 2
OH 3 7 3 47 3 55 3 49
F 3 95 3 95 3 75
Cl 3 03 3 05 2 68
Br 2 80 2 75
I 2 28
a. P. R. Wells, Prog. Phys. Org. Chem., 6, 111 (1968).
b. N. Inamoto and S. Masuda, Chem. Lett., 1003 (1982).
c. R. J. Boyd and S. L. Boyd, J. Am. Chem. Soc., 114, 1652 (1992).
d. S. Hati and D. Datta, J. Comput. Chem., 13, 912 (1992).
the theoretical results pertain to isolated molecules, whereas the empirical values are
derived from measurements in solution. The smaller size of the methyl group makes
it less polarizable than larger alkyl groups. This difference is maximized in the gas
phase, where there are no compensating solvent effects.
Let us now consider a few simple but important systems that can illustrate the
application of these ideas. We know that the acidity of the hydrides of the second-row
elements increases sharply going to the right in the periodic table. The same trend is
true for the third row and in each case the third-row compound is more acidic than its
second-row counterpart. This is illustrated by the gas phase ionization enthalpy data
in Table 1.28.
The gas phase data from both the second- and third-row compounds can be
correlated by an expression that contains terms both for electronegativity and
hardness % . The sign is negative for hardness:
G = 311 805−18 118 +33 771% (1.50)
acid
Table 1.27. Gas Phase and Aqueous pK a
Values
pK a pK a
FCH 2 CO 2 H 2.59 ClCH 2 CO 2 H 2 87
CHF 2 CO 2 H NA CHCl 2 CO 2 H 1 35
CF 3 CO 2 H 0.52 CCl 3 CO 2 H 0 66