Page 128 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 128
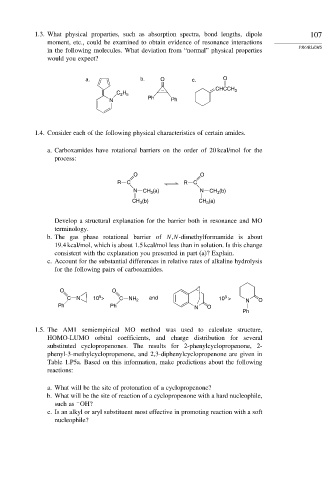
1.3. What physical properties, such as absorption spectra, bond lengths, dipole 107
moment, etc., could be examined to obtain evidence of resonance interactions
PROBLEMS
in the following molecules. What deviation from “normal” physical properties
would you expect?
a. b. O c. O
CHCCH 3
H
C 2 5
Ph
N Ph
1.4. Consider each of the following physical characteristics of certain amides.
a. Carboxamides have rotational barriers on the order of 20 kcal/mol for the
process:
O O
R C R C
N CH (a) N CH (b)
3
3
CH (b) CH (a)
3
3
Develop a structural explanation for the barrier both in resonance and MO
terminology.
b. The gas phase rotational barrier of N,N-dimethylformamide is about
19.4 kcal/mol, which is about 1.5 kcal/mol less than in solution. Is this change
consistent with the explanation you presented in part (a)? Explain.
c. Account for the substantial differences in relative rates of alkaline hydrolysis
for the following pairs of carboxamides.
O O
5
3
C N 10 > C NH 2 and 10 > N O
Ph Ph N O
Ph
1.5. The AM1 semiempirical MO method was used to calculate structure,
HOMO-LUMO orbital coefficients, and charge distribution for several
substituted cyclopropenones. The results for 2-phenylcyclopropenone, 2-
phenyl-3-methylcyclopropenone, and 2,3-diphenylcyclopropenone are given in
Table 1.P5a. Based on this information, make predictions about the following
reactions:
a. What will be the site of protonation of a cyclopropenone?
b. What will be the site of reaction of a cyclopropenone with a hard nucleophile,
such as OH?
−
c. Is an alkyl or aryl substituent most effective in promoting reaction with a soft
nucleophile?