Page 131 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 131
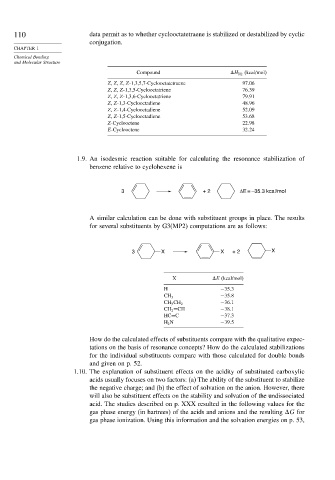
110 data permit as to whether cyclooctatetraene is stabilized or destabilized by cyclic
conjugation.
CHAPTER 1
Chemical Bonding
and Molecular Structure
Compound
H H2 (kcal/mol)
Z Z Z Z-1,3,5,7-Cyclooctatetraene 97 06
Z Z Z-1,3,5-Cyclooctatriene 76 39
Z Z Z-1,3,6-Cyclooctatriene 79 91
Z Z-1,3-Cyclooctadiene 48 96
Z Z-1,4-Cyclooctadiene 52 09
Z Z-1,5-Cyclooctadiene 53 68
Z-Cyclooctene 22 98
E-Cyclooctene 32 24
1.9. An isodesmic reaction suitable for calculating the resonance stabilization of
benzene relative to cyclohexene is
3 + 2 ΔE = –35.3 kcal/mol
A similar calculation can be done with substituent groups in place. The results
for several substituents by G3(MP2) computations are as follows:
3 X X + 2 X
X
E (kcal/mol)
H −35 3
−35 8
CH 3
−36 1
CH 3 CH 2
CH 2 =CH −38 1
HC≡C −37 3
H 2 N −39 5
How do the calculated effects of substituents compare with the qualitative expec-
tations on the basis of resonance concepts? How do the calculated stabilizations
for the individual substituents compare with those calculated for double bonds
and given on p. 52.
1.10. The explanation of substituent effects on the acidity of substituted carboxylic
acids usually focuses on two factors: (a) The ability of the substituent to stabilize
the negative charge; and (b) the effect of solvation on the anion. However, there
will also be substituent effects on the stability and solvation of the undissociated
acid. The studies described on p. XXX resulted in the following values for the
gas phase energy (in hartrees) of the acids and anions and the resulting
G for
gas phase ionization. Using this information and the solvation energies on p. 53,