Page 130 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 130
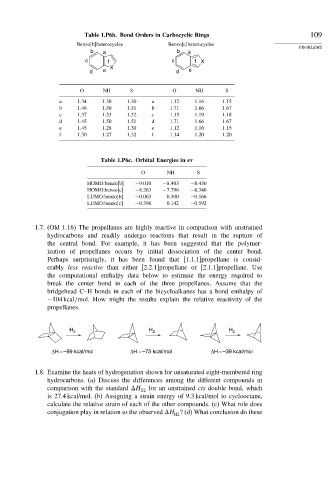
Table 1.P6b. Bond Orders in Carbocyclic Rings 109
Benzo[b]heterocycles Benzo[c]heterocycles
PROBLEMS
b a b a
c f c f X
X
d e d e
O NH S O NH S
a 1 34 1 30 1 30 a 1 12 1 16 1 15
b 1 46 1 50 1 51 b 1 71 1 66 1 67
c 1 37 1 33 1 32 c 1 15 1 19 1 18
d 1 45 1 50 1 51 d 1 71 1 66 1 67
e 1 45 1 28 1 30 e 1 12 1 16 1 15
f 1 30 1 27 1 32 f 1 14 1 20 1 20
Table 1.P6c. Orbital Energies in ev
O NH S
HOMO:benzo[b] −9 010 −8 403 −8 430
HOMO:benzo[c] −8 263 −7 796 −8 340
LUMO:benzo[b] −0 063 0.300 −0 166
LUMO:benzo[c] −0 396 0.142 −0 592
1.7. (Old 1.16) The propellanes are highly reactive in comparison with unstrained
hydrocarbons and readily undergo reactions that result in the rupture of
the central bond. For example, it has been suggested that the polymer-
ization of propellanes occurs by initial dissociation of the center bond.
Perhaps surprisingly, it has been found that [1.1.1]propellane is consid-
erably less reactive than either [2.2.1]propellane or [2.1.1]propellane. Use
the computational enthalpy data below to estimate the energy required to
break the center bond in each of the three propellanes. Assume that the
bridgehead C–H bonds in each of the bicycloalkanes has a bond enthalpy of
−104kcal/mol. How might the results explain the relative reactivity of the
propellanes.
H 2 H 2 H 2
ΔH = –99 kcal/mol ΔH = –73 kcal/mol ΔH = –39 kcal/mol
1.8. Examine the heats of hydrogenation shown for unsaturated eight-membered ring
hydrocarbons. (a) Discuss the differences among the different compounds in
comparison with the standard
H for an unstrained cis double bond, which
H2
is 27.4 kcal/mol. (b) Assigning a strain energy of 9.3 kcal/mol to cyclooctane,
calculate the relative strain of each of the other compounds. (c) What role does
conjugation play in relation to the observed
H ? (d) What conclusion do these
H2