Page 125 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 125
104
CHAPTER 1
Chemical Bonding
and Molecular Structure
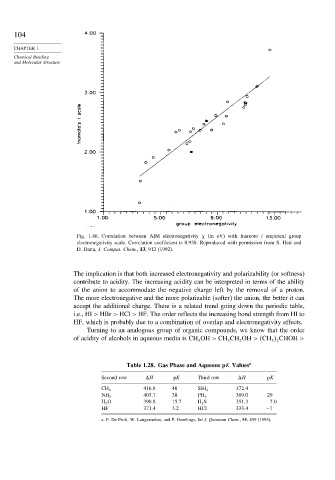
Fig. 1.46. Correlation between AIM electronegativity (in eV) with Inamoto i empirical group
electronegativity scale. Correlation coefficient is 0.938. Reproduced with permission from S. Hati and
D. Datta, J. Comput. Chem., 13, 912 (1992).
The implication is that both increased electronegativity and polarizability (or softness)
contribute to acidity. The increasing acidity can be interpreted in terms of the ability
of the anion to accommodate the negative charge left by the removal of a proton.
The more electronegative and the more polarizable (softer) the anion, the better it can
accept the additional charge. There is a related trend going down the periodic table,
i.e., HI > HBr > HCl > HF. The order reflects the increasing bond strength from HI to
HF, which is probably due to a combination of overlap and electronegativity effects.
Turning to an analogous group of organic compounds, we know that the order
of acidity of alcohols in aqueous media is CH OH > CH CH OH > CH CHOH >
3 3 2 3 2
Table 1.28. Gas Phase and Aqueous pK Values a
Second row
H pK Third row
H pK
416 8 48 372 4
CH 4 SiH 4
403 7 38 369 0 29
NH 3 PH 3
H 2 O 390 8 15.7 H 2 S 351 3 7 0
HF 371 4 3.2 HCl 333 4 −7
a. F. De Proft, W. Langenaeker, and P. Geerlings, Int J. Quantum Chem., 55, 459 (1995).