Page 123 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 123
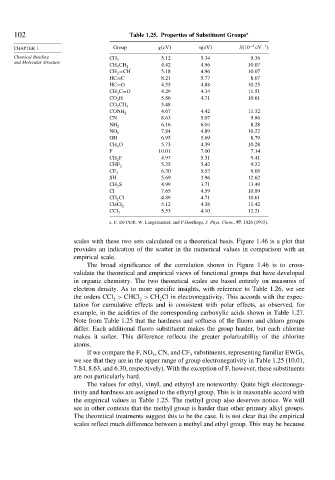
102 Table 1.25. Properties of Substituent Groups a
−1
CHAPTER 1 Group eV eV S 10 −2 eV
Chemical Bonding 5 12 5.34 9 36
CH 3
and Molecular Structure
CH 3 CH 2 4 42 4.96 10 07
CH 2 =CH 5 18 4.96 10 07
HC≡C 8 21 5.77 8 67
HC=O 4 55 4.88 10 25
CH 3 C=O 4 29 4.34 11 51
CO 2 H 5 86 4.71 10 61
5 48
CO 2 CH 3
4 67 4.42 11 32
CONH 2
CN 8 63 5.07 9 86
6 16 6.04 8 28
NH 2
7 84 4.89 10 22
NO 2
OH 6 95 5.69 8 79
CH 3 O 5 73 4.39 10 28
F 10 01 7.00 7 14
CH 2 F 4 97 5.31 9 41
5 25 5.42 9 22
CHF 2
6 30 5.53 9 05
CF 3
SH 5 69 3.96 12 62
CH 3 S 4 99 3.71 13 49
Cl 7 65 4.59 10 89
CH 2 Cl 4 89 4.71 10 61
5 12 4.38 11 42
CHCl 2
5 53 4.10 12 21
CCl 3
a. F. De Proft, W. Langemaeker, and P.Geerlings, J. Phys. Chem., 97, 1826 (1993).
scales with these two sets calculated on a theoretical basis. Figure 1.46 is a plot that
provides an indication of the scatter in the numerical values in comparison with an
empirical scale.
The broad significance of the correlation shown in Figure 1.46 is to cross-
validate the theoretical and empirical views of functional groups that have developed
in organic chemistry. The two theoretical scales are based entirely on measures of
electron density. As to more specific insights, with reference to Table 1.26, we see
the orders CCl > CHCl > CH Cl in electronegativity. This accords with the expec-
3 2 2
tation for cumulative effects and is consistent with polar effects, as observed, for
example, in the acidities of the corresponding carboxylic acids shown in Table 1.27.
Note from Table 1.25 that the hardness and softness of the fluoro and chloro groups
differ. Each additional fluoro substituent makes the group harder, but each chlorine
makes it softer. This difference reflects the greater polarizability of the chlorine
atoms.
If we compare the F, NO , CN, and CF substituents, representing familiar EWGs,
3
2
we see that they are in the upper range of group electronegativity in Table 1.25 (10.01,
7.84, 8.63, and 6.30, respectively). With the exception of F, however, these substituents
are not particularly hard.
The values for ethyl, vinyl, and ethynyl are noteworthy. Quite high electronega-
tivity and hardness are assigned to the ethynyl group. This is in reasonable accord with
the empirical values in Table 1.25. The methyl group also deserves notice. We will
see in other contexts that the methyl group is harder than other primary alkyl groups.
The theoretical treatments suggest this to be the case. It is not clear that the empirical
scales reflect much difference between a methyl and ethyl group. This may be because