Page 111 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 111
90 Surprisingly, [1.1.1]propellane is somewhat more stable to thermal decomposition
than the next larger propellane, [2.1.1]propellane, indicating a reversal in the trend of
CHAPTER 1 increased reactivity with increased strain. To understand this observation, it is important
Chemical Bonding to recognized that the energy of both the reactant and intermediate influence the rate of
and Molecular Structure
unimolecular reactions that lead to decomposition. In the case of propellanes, homolytic
rupture of the central bond is expected to be the initial step in decomposition. This
bond rupture is very endothermic for [1.1.1]propellane. Because relatively less strain
is released in the case of [1.1.1]propellane than in the [2.1.1]- and [2.2.1]-homologs,
[1.1.1]propellane is kinetically most stable. 134
ΔH = +65 kcal/mol +30 kcal/mol +5 kcal/mol
Another manifestation of the relatively small release of strain associated with breaking
the central bond comes from MP4/6-31G calculations on the energy of the reverse
∗
ring closure. 135
H
+ H
+ 27 kcal/mol
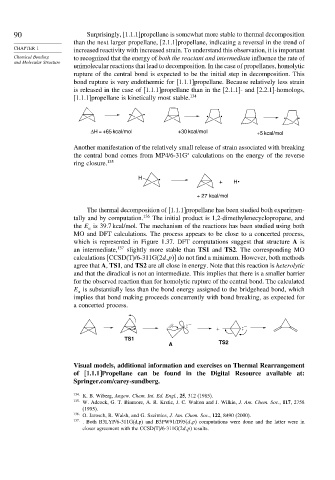
The thermal decomposition of [1.1.1]propellane has been studied both experimen-
tally and by computation. 136 The initial product is 1,2-dimethylenecyclopropane, and
the E is 39.7 kcal/mol. The mechanism of the reactions has been studied using both
a
MO and DFT calculations. The process appears to be close to a concerted process,
which is represented in Figure 1.37. DFT computations suggest that structure A is
an intermediate, 137 slightly more stable than TS1 and TS2. The corresponding MO
calculations [CCSD(T)/6-311G(2d,p)] do not find a minimum. However, both methods
agree that A, TS1, and TS2 are all close in energy. Note that this reaction is heterolytic
and that the diradical is not an intermediate. This implies that there is a smaller barrier
for the observed reaction than for homolytic rupture of the central bond. The calculated
E is substantially less than the bond energy assigned to the bridgehead bond, which
a
implies that bond making proceeds concurrently with bond breaking, as expected for
a concerted process.
– –
+
TS1
A TS2
Visual models, additional information and exercises on Thermal Rearrangement
of [1.1.1]Propellane can be found in the Digital Resource available at:
Springer.com/carey-sundberg.
134
K. B. Wiberg, Angew. Chem. Int. Ed. Engl., 25, 312 (1985).
135 W. Adcock, G. T. Binmore, A. R. Krstic, J. C. Walton and J. Wilkie, J. Am. Chem. Soc., 117, 2758
(1995).
136 O. Jarosch, R. Walsh, and G. Szeimies, J. Am. Chem. Soc., 122, 8490 (2000).
137
. Both B3LYP/6-311G(d,p) and B3PW91/D95(d,p) computations were done and the latter were in
closer agreement with the CCSD(T)/6-311G(2d,p) results.