Page 108 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 108
87
TOPIC 1.3
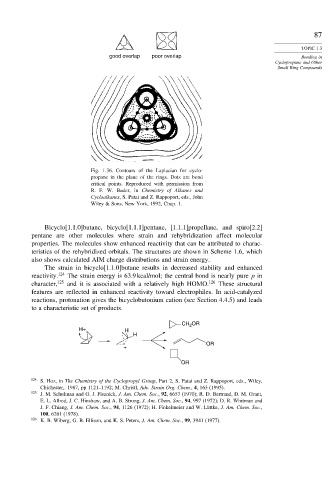
good overlap poor overlap Bonding in
Cyclopropane and Other
Small Ring Compounds
Fig. 1.36. Contours of the Laplacian for cyclo-
propane in the plane of the rings. Dots are bond
critical points. Reproduced with permission from
R. F. W. Bader, in Chemistry of Alkanes and
Cycloalkanes, S. Patai and Z. Rappoport, eds., John
Wiley & Sons, New York, 1992, Chap. 1.
Bicyclo[1.1.0]butane, bicyclo[1.1.1]pentane, [1.1.1]propellane, and spiro[2.2]
pentane are other molecules where strain and rehybridization affect molecular
properties. The molecules show enhanced reactivity that can be attributed to charac-
teristics of the rehybridized orbitals. The structures are shown in Scheme 1.6, which
also shows calculated AIM charge distributions and strain energy.
The strain in bicyclo[1.1.0]butane results in decreased stability and enhanced
reactivity. 124 The strain energy is 63.9 kcal/mol; the central bond is nearly pure p in
character, 125 and it is associated with a relatively high HOMO. 126 These structural
features are reflected in enhanced reactivity toward electrophiles. In acid-catalyzed
reactions, protonation gives the bicyclobutonium cation (see Section 4.4.5) and leads
to a characteristic set of products.
CH OR
2
H+ H
H
OR
+
OR
124 S. Hoz, in The Chemistry of the Cyclopropyl Group, Part 2, S. Patai and Z. Rappoport, eds., Wiley,
Chichester,. 1987, pp 1121–1192; M. Christl, Adv. Strain Org. Chem., 4, 163 (1995).
125 J. M. Schulman and G. J. Fisanick, J. Am. Chem. Soc., 92, 6653 (1970); R. D. Bertrand, D. M. Grant,
E. L. Allred, J. C. Hinshaw, and A. B. Strong, J. Am. Chem. Soc., 94, 997 (1972); D. R. Whitman and
J. F. Chiang, J. Am. Chem. Soc., 94, 1126 (1972); H. Finkelmeier and W. Lüttke, J. Am. Chem. Soc.,
100, 6261 (1978).
126
K. B. Wiberg, G. B. Ellison, and K. S. Peters, J. Am. Chem. Soc., 99, 3941 (1977).