Page 104 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 104
–
+
H 2 N– CH 2 X ↔ H 2 N =CH 2 X − 83
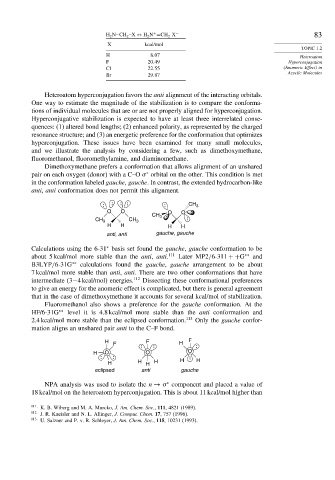
X kcal/mol
TOPIC 1.2
H 8 07 Heteroatom
F 20 49 Hyperconjugation
Cl 22 55 (Anomeric Effect) in
Acyclic Molecules
Br 29 87
Heteroatom hyperconjugation favors the anti alignment of the interacting orbitals.
One way to estimate the magnitude of the stabilization is to compare the conforma-
tions of individual molecules that are or are not properly aligned for hyperconjugation.
Hyperconjugative stabilization is expected to have at least three interrelated conse-
quences: (1) altered bond lengths; (2) enhanced polarity, as represented by the charged
resonance structure; and (3) an energetic preference for the conformation that optimizes
hyperconjugation. These issues have been examined for many small molecules,
and we illustrate the analysis by considering a few, such as dimethoxymethane,
fluoromethanol, fluoromethylamine, and diaminomethane.
Dimethoxymethane prefers a conformation that allows alignment of an unshared
pair on each oxygen (donor) with a C–O orbital on the other. This condition is met
∗
in the conformation labeled gauche, gauche. In contrast, the extended hydrocarbon-like
anti, anti conformation does not permit this alignment.
CH 3
O O O O
CH 3
CH 3 CH 3
H H H H
anti, anti gauche, gauche
∗
Calculations using the 6-31 basis set found the gauche, gauche conformation to be
about 5 kcal/mol more stable than the anti, anti. 111 Later MP2/6-311 ++G ∗∗ and
B3LYP/6-31G ∗∗ calculations found the gauche, gauche arrangement to be about
7 kcal/mol more stable than anti, anti. There are two other conformations that have
intermediate (3−4 kcal/mol) energies. 112 Dissecting these conformational preferences
to give an energy for the anomeric effect is complicated, but there is general agreement
that in the case of dimethoxymethane it accounts for several kcal/mol of stabilization.
Fluoromethanol also shows a preference for the gauche conformation. At the
HF/6-31G ∗∗ level it is 4.8 kcal/mol more stable than the anti conformation and
2.4 kcal/mol more stable than the eclipsed conformation. 113 Only the gauche confor-
mation aligns an unshared pair anti to the C–F bond.
H F F
F H
H O O O
H H H H H H
eclipsed anti gauche
∗
NPA analysis was used to isolate the n → component and placed a value of
18 kcal/mol on the heteroatom hyperconjugation. This is about 11 kcal/mol higher than
111
K. B. Wiberg and M. A. Murcko, J. Am. Chem. Soc., 111, 4821 (1989).
112 J. R. Kneisler and N. L. Allinger, J. Comput. Chem. 17, 757 (1996).
113
U. Salzner and P. v. R. Schleyer, J. Am. Chem. Soc., 115, 10231 (1993).