Page 426 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 426
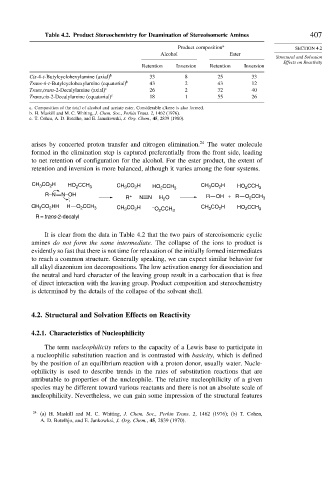
Table 4.2. Product Stereochemistry for Deamination of Stereoisomeric Amines 407
Product composition a SECTION 4.2
Alcohol Ester
Structural and Solvation
Effects on Reactivity
Retention Inversion Retention Inversion
Cis-4-t-Butylcyclohexylamine (axial) b 33 8 25 33
Trans-4-t-Butylcyclohexylamine (equatorial) b 43 2 43 12
Trans,trans-2-Decalylamine (axial) c 26 2 32 40
Trans,cis-2-Decalylamine (equatorial) c 18 1 55 26
a. Composition of the total of alcohol and acetate ester. Considerable alkene is also formed.
b. H. Maskill and M. C. Whiting, J. Chem. Soc., Perkin Trans. 2, 1462 (1976).
c. T. Cohen, A. D. Botelho, and E. Jamnkowski, J. Org. Chem., 45, 2839 (1980).
arises by concerted proton transfer and nitrogen elimination. 24 The water molecule
formed in the elimination step is captured preferentially from the front side, leading
to net retention of configuration for the alcohol. For the ester product, the extent of
retention and inversion is more balanced, although it varies among the four systems.
CH CO H HO CCH 3 CH CO H HO 2 CCH 3 CH 3 CO 2 H HO CCH 3
3
2
2
2
2
3
R–N N–OH + R OH + R O CCH
R NN H O 2 3
2
CH CO HH H O CCH 3 CH CO H – O CCH 3 CH 3 CO H HO CCH 3
3
2
2
2
2
2
3
2
R = trans-2-decalyl
It is clear from the data in Table 4.2 that the two pairs of stereoisomeric cyclic
amines do not form the same intermediate. The collapse of the ions to product is
evidently so fast that there is not time for relaxation of the initially formed intermediates
to reach a common structure. Generally speaking, we can expect similar behavior for
all alkyl diazonium ion decompositions. The low activation energy for dissociation and
the neutral and hard character of the leaving group result in a carbocation that is free
of direct interaction with the leaving group. Product composition and stereochemistry
is determined by the details of the collapse of the solvent shell.
4.2. Structural and Solvation Effects on Reactivity
4.2.1. Characteristics of Nucleophilicity
The term nucleophilicity refers to the capacity of a Lewis base to participate in
a nucleophilic substitution reaction and is contrasted with basicity, which is defined
by the position of an equilibrium reaction with a proton donor, usually water. Nucle-
ophilicity is used to describe trends in the rates of substitution reactions that are
attributable to properties of the nucleophile. The relative nucleophilicity of a given
species may be different toward various reactants and there is not an absolute scale of
nucleophilicity. Nevertheless, we can gain some impression of the structural features
24
(a) H. Maskill and M. C. Whiting, J. Chem. Soc., Perkin Trans. 2, 1462 (1976); (b) T. Cohen,
A. D. Botelhjo, and E. Jankowksi, J. Org. Chem., 45, 2839 (1970).