Page 424 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 424
molecule and the anion of the ion pair facilitates capture of a water molecule from the 405
front side of the ion pair.
SECTION 4.1
H Mechanisms for
CH CH 3 Nucleophilic Substitution
3
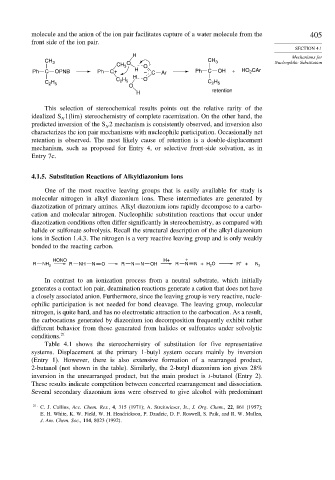
CH 3 O O
Ph C OPNB Ph C+ H – C Ar Ph C OH + HO CAr
2
H
C H O
H 2 5 C H
C 2 5 2 5
O
retention
H
This selection of stereochemical results points out the relative rarity of the
idealized S 1 lim stereochemistry of complete racemization. On the other hand, the
N
predicted inversion of the S 2 mechanism is consistently observed, and inversion also
N
characterizes the ion pair mechanisms with nucleophile participation. Occasionally net
retention is observed. The most likely cause of retention is a double-displacement
mechanism, such as proposed for Entry 4, or selective front-side solvation, as in
Entry 7c.
4.1.5. Substitution Reactions of Alkyldiazonium Ions
One of the most reactive leaving groups that is easily available for study is
molecular nitrogen in alkyl diazonium ions. These intermediates are generated by
diazotization of primary amines. Alkyl diazonium ions rapidly decompose to a carbo-
cation and molecular nitrogen. Nucleophilic substitution reactions that occur under
diazotization conditions often differ significantly in stereochemistry, as compared with
halide or sulfonate solvolysis. Recall the structural description of the alkyl diazonium
ions in Section 1.4.3. The nitrogen is a very reactive leaving group and is only weakly
bonded to the reacting carbon.
HONO H+ +
R NH 2 R NH N O R N N OH R N N + H O R + + N 2
2
In contrast to an ionization process from a neutral substrate, which initially
generates a contact ion pair, deamination reactions generate a cation that does not have
a closely associated anion. Furthermore, since the leaving group is very reactive, nucle-
ophilic participation is not needed for bond cleavage. The leaving group, molecular
nitrogen, is quite hard, and has no electrostatic attraction to the carbocation. As a result,
the carbocations generated by diazonium ion decomposition frequently exhibit rather
different behavior from those generated from halides or sulfonates under solvolytic
conditions. 21
Table 4.1 shows the stereochemistry of substitution for five representative
systems. Displacement at the primary 1-butyl system occurs mainly by inversion
(Entry 1). However, there is also extensive formation of a rearranged product,
2-butanol (not shown in the table). Similarly, the 2-butyl diazonium ion gives 28%
inversion in the unrearranged product, but the main product is t-butanol (Entry 2).
These results indicate competition between concerted rearrangement and dissociation.
Several secondary diazonium ions were observed to give alcohol with predominant
21
C. J. Collins, Acc. Chem. Res., 4, 315 (1971); A. Streitwieser, Jr., J. Org. Chem., 22, 861 (1957);
E. H. White, K. W. Field, W. H. Hendrickson, P. Dzadzic, D. F. Roswell, S. Paik, and R. W. Mullen,
J. Am. Chem. Soc., 114, 8023 (1992).