Page 422 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 422
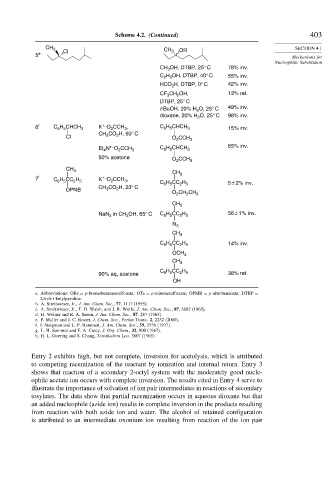
Scheme 4.2. (Continued) 403
CH 3 CH OR SECTION 4.1
5 e Cl 3
Mechanisms for
Nucleophilic Substitution
CH OH, DTBP, 25° C 78% inv.
3
C H OH, DTBP, 40° C 55% inv.
2 5
HCO H, DTBP, 0° C 42% inv.
2
CF CH OH, 13% ret.
2
3
DTBP, 25° C
t-BuOH, 20% H 2 O, 25° C 49% inv.
O, 25° C 98% inv.
dioxane, 20% H 2
+
H CHCH
6 f C H CHCH 3 K – O CCH , C 6 5 3 15% inv.
3
2
6 5
CH CO H, 50° C
Cl 3 2 O CCH 3
2
+
N – O CCH C H CHCH 65% inv.
Et 4 2 3 6 5 3
50% acetone
O 2 CCH 3
CH 3 CH
3
+
7 f C H CC H K – O CCH , C H CC H 5 ± 2% inv.
2
3
2 5
6 5
CH CO H, 23° C 6 5 2 5
OPNB 3 2
O CH CH 3
2
2
CH 3
NaN in CH 3 OH, 65° C C 6 H 5 CC 2 H 5 56 ± 1% inv.
3
N 3
CH 3
C H CC H 14% inv.
6 5
2 5
OCH 3
CH 3
90% aq, acetone C 6 H 5 CC 2 H 5 38% ret.
OH
a. Abbreviations: OBs = p-bromobenzenesulfonate; OTs = p-toluenesulfonate; OPMB = p-nitrobenzoate; DTBP =
2,6-di-t-butylpyridine.
b. A. Streitwieser, Jr., J. Am. Chem. Soc., 77, 1117 (1955).
c. A. Streitwieser, Jr., T. D. Walsh, and J. R. Wolfe, J. Am. Chem. Soc., 87, 3682 (1965).
d. H. Weiner and R. A. Sneen, J. Am. Chem. Soc., 87, 287 (1965).
e. P. Muller and J. C. Rosier, J. Chem. Soc., Perkin Trans., 2, 2232 (2000).
f. J. Steigman and L. P. Hammett, J. Am. Chem. Soc., 59, 2536 (1937).
g. L. H. Sommer and F. A. Carey, J. Org. Chem., 32, 800 (1967).
h. H. L. Goering and S. Chang, Tetrahedron Lett. 3607 (1965).
Entry 2 exhibits high, but not complete, inversion for acetolysis, which is attributed
to competing racemization of the reactant by ionization and internal return. Entry 3
shows that reaction of a secondary 2-octyl system with the moderately good nucle-
ophile acetate ion occurs with complete inversion. The results cited in Entry 4 serve to
illustrate the importance of solvation of ion pair intermediates in reactions of secondary
tosylates. The data show that partial racemization occurs in aqueous dioxane but that
an added nucleophile (azide ion) results in complete inversion in the products resulting
from reaction with both azide ion and water. The alcohol of retained configuration
is attributed to an intermediate oxonium ion resulting from reaction of the ion pair