Page 420 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 420
401
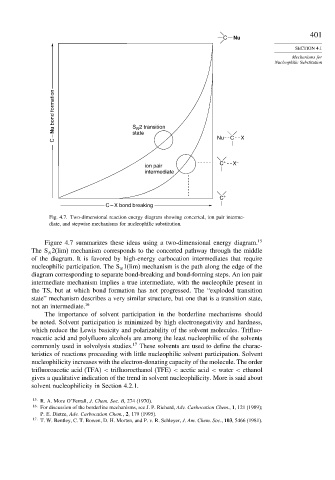
C Nu
SECTION 4.1
Mechanisms for
Nucleophilic Substitution
C – Nu bond formation S 2 transition Nu C X
N
state
ion pair C + X –
intermediate
C +
C – X bond breaking
Fig. 4.7. Two-dimensional reaction energy diagram showing concerted, ion pair interme-
diate, and stepwise mechanisms for nucleophilic substitution.
Figure 4.7 summarizes these ideas using a two-dimensional energy diagram. 15
The S 2(lim) mechanism corresponds to the concerted pathway through the middle
N
of the diagram. It is favored by high-energy carbocation intermediates that require
nucleophilic participation. The S 1(lim) mechanism is the path along the edge of the
N
diagram corresponding to separate bond-breaking and bond-forming steps. An ion pair
intermediate mechanism implies a true intermediate, with the nucleophile present in
the TS, but at which bond formation has not progressed. The “exploded transition
state” mechanism describes a very similar structure, but one that is a transition state,
not an intermediate. 16
The importance of solvent participation in the borderline mechanisms should
be noted. Solvent participation is minimized by high electronegativity and hardness,
which reduce the Lewis basicity and polarizability of the solvent molecules. Trifluo-
roacetic acid and polyfluoro alcohols are among the least nucleophilic of the solvents
commonly used in solvolysis studies. 17 These solvents are used to define the charac-
teristics of reactions proceeding with little nucleophilic solvent participation. Solvent
nucleophilicity increases with the electron-donating capacity of the molecule. The order
trifluoroacetic acid (TFA) < trifluoroethanol (TFE) < acetic acid < water < ethanol
gives a qualitative indication of the trend in solvent nucleophilicity. More is said about
solvent nucleophilicity in Section 4.2.1.
15 R. A. More O’Ferrall, J. Chem. Soc. B, 274 (1970).
16 For discussion of the borderline mechanisms, see J. P. Richard, Adv. Carbocation Chem., 1, 121 (1989);
P. E. Dietze, Adv. Carbocation Chem., 2, 179 (1995).
17
T. W. Bentley, C. T. Bowen, D. H. Morten, and P. v. R. Schleyer, J. Am. Chem. Soc., 103, 5466 (1981).