Page 416 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 416
reactant more rapidly than it is captured by azide ion, whereas the solvent-separated 397
ion pair is captured by azide ion faster than it returns to the racemic reactant.
SECTION 4.1
k ex k rac Mechanisms for
+ –
Ar 2 CHO 2 CAr' [Ar 2 CH + – O 2 CAr'] [Ar 2 CH // O 2 CAr'] Ar 2 CHO 2 CAr' Nucleophilic Substitution
–
N 3
Ar CHN 3
2
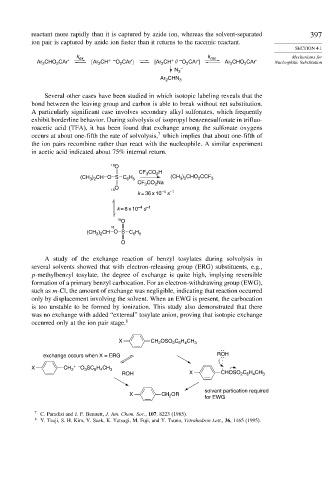
Several other cases have been studied in which isotopic labeling reveals that the
bond between the leaving group and carbon is able to break without net substitution.
A particularly significant case involves secondary alkyl sulfonates, which frequently
exhibit borderline behavior. During solvolysis of isopropyl benzenesulfonate in trifluo-
roacetic acid (TFA), it has been found that exchange among the sulfonate oxygens
7
occurs at about one-fifth the rate of solvolysis, which implies that about one-fifth of
the ion pairs recombine rather than react with the nucleophile. A similar experiment
in acetic acid indicated about 75% internal return.
18
O
CF CO H
2
3
) CHO CCF
H
(CH ) CH O S C 6 5 (CH 3 2 2 3
3 2
CO Na
CF 3 2
O
18 –4 –1
k = 36 x 10 s
s
k = 8 x 10 –4 –1
18 O
18
) CH O S C H
(CH 3 2 6 5
O
A study of the exchange reaction of benzyl tosylates during solvolysis in
several solvents showed that with electron-releasing group (ERG) substituents, e.g.,
p-methylbenzyl tosylate, the degree of exchange is quite high, implying reversible
formation of a primary benzyl carbocation. For an electron-withdrawing group (EWG),
such as m-Cl, the amount of exchange was negligible, indicating that reaction occurred
only by displacement involving the solvent. When an EWG is present, the carbocation
is too unstable to be formed by ionization. This study also demonstrated that there
was no exchange with added “external” tosylate anion, proving that isotopic exchange
occurred only at the ion pair stage. 8
X CH OSO C H CH 3
2 6 4
2
exchange occurs when X = ERG ROH
X CH 2 + – O SC H CH 3
3
6 4
ROH X CHOSO C H CH 3
2 6 4
solvent partication required
X CH OR for EWG
2
7 C. Paradisi and J. F. Bunnett, J. Am. Chem. Soc., 107, 8223 (1985).
8
Y. Tsuji, S. H. Kim, Y. Saek, K. Yatsugi, M. Fuji, and Y. Tsuno, Tetrahedron Lett., 36, 1465 (1995).