Page 417 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 417
398 The ion pair return phenomenon can also be demonstrated by comparing the rate
of racemization of reactant with the rate of product formation. For a number of systems,
CHAPTER 4 9
including l-arylethyl tosylates, the rate of decrease of optical rotation is greater than
Nucleophilic Substitution the rate of product formation, which indicates the existence of an intermediate that can
re-form racemic reactant. The solvent-separated ion pair is the most likely intermediate
to play this role.
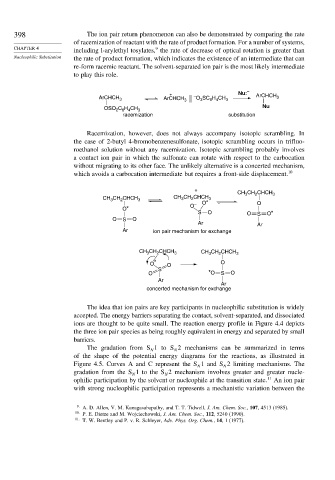
+ Nu: – ArCHCH
ArCHCH 3 ArCHCH 3 – O SC H CH 3 3
3
6 4
OSO C H CH 3 Nu
2 6 4
racemization substitution
Racemization, however, does not always accompany isotopic scrambling. In
the case of 2-butyl 4-bromobenzenesulfonate, isotopic scrambling occurs in trifluo-
roethanol solution without any racemization. Isotopic scrambling probably involves
a contact ion pair in which the sulfonate can rotate with respect to the carbocation
without migrating to its other face. The unlikely alternative is a concerted mechanism,
which avoids a carbocation intermediate but requires a front-side displacement. 10
+
2
CH 3 CH CHCH 3
CH CH CHCH 3 CH 3 CH CHCH 3 O
2
3
2
O*
O* O –
S O O S O*
O S O
Ar Ar
Ar ion pair mechanism for exchange
CH CH CHCH 3 CH CH CHCH 3
2
3
3
2
* O
O O
S
O *O S O
Ar
Ar
concerted mechanism for exchange
The idea that ion pairs are key participants in nucleophilic substitution is widely
accepted. The energy barriers separating the contact, solvent-separated, and dissociated
ions are thought to be quite small. The reaction energy profile in Figure 4.4 depicts
the three ion pair species as being roughly equivalent in energy and separated by small
barriers.
The gradation from S 1toS 2 mechanisms can be summarized in terms
N
N
of the shape of the potential energy diagrams for the reactions, as illustrated in
Figure 4.5. Curves A and C represent the S 1 and S 2 limiting mechanisms. The
N
N
gradation from the S 1 to the S 2 mechanism involves greater and greater nucle-
N
N
11
ophilic participation by the solvent or nucleophile at the transition state. An ion pair
with strong nucleophilic participation represents a mechanistic variation between the
9
A. D. Allen, V. M. Kanagasabapathy, and T. T. Tidwell, J. Am. Chem. Soc., 107, 4513 (1985).
10 P. E. Dietze and M. Wojciechowski, J. Am. Chem. Soc., 112, 5240 (1990).
11
T. W. Bentley and P. v. R. Schleyer, Adv. Phys. Org. Chem., 14, 1 (1977).