Page 419 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 419
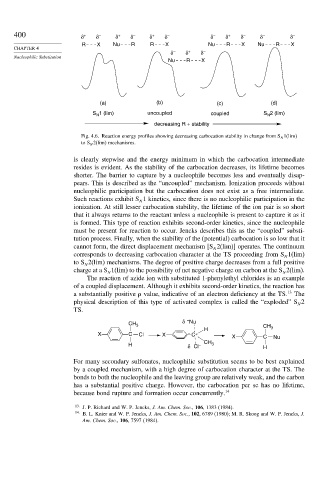
400 δ + δ – δ + δ – δ + δ – δ – δ + δ – δ – δ –
R X Nu R R X Nu R X Nu R X
CHAPTER 4
δ – δ + δ –
Nucleophilic Substitution
Nu R X
(a) (b) (c) (d)
S 1 (lim) uncoupled coupled S 2 (lim)
N
N
decreasing R + stability
Fig. 4.6. Reaction energy profiles showing decreasing carbocation stability in change from S N 1(lim)
to S N 2(lim) mechanisms.
is clearly stepwise and the energy minimum in which the carbocation intermediate
resides is evident. As the stability of the carbocation decreases, its lifetime becomes
shorter. The barrier to capture by a nucleophile becomes less and eventually disap-
pears. This is described as the “uncoupled” mechanism. Ionization proceeds without
nucleophilic participation but the carbocation does not exist as a free intermediate.
Such reactions exhibit S 1 kinetics, since there is no nucleophilic participation in the
N
ionization. At still lesser carbocation stability, the lifetime of the ion pair is so short
that it always returns to the reactant unless a nucleophile is present to capture it as it
is formed. This type of reaction exhibits second-order kinetics, since the nucleophile
must be present for reaction to occur. Jencks describes this as the “coupled” substi-
tution process. Finally, when the stability of the (potential) carbocation is so low that it
cannot form, the direct displacement mechanism [S 2(lim)] operates. The continuum
N
corresponds to decreasing carbocation character at the TS proceeding from S 1(lim)
N
to S 2(lim) mechanisms. The degree of positive charge decreases from a full positive
N
charge at a S 1(lim) to the possibility of net negative charge on carbon at the S 2(lim).
N N
The reaction of azide ion with substituted 1-phenylethyl chlorides is an example
of a coupled displacement. Although it exhibits second-order kinetics, the reaction has
a substantially positive
value, indicative of an electron deficiency at the TS. 13 The
physical description of this type of activated complex is called the “exploded” S 2
N
TS.
δ – Nu
CH 3 CH
H 3
X C Cl X C + X C Nu
H δ Cl – CH 3 H
For many secondary sulfonates, nucleophilic substitution seems to be best explained
by a coupled mechanism, with a high degree of carbocation character at the TS. The
bonds to both the nucleophile and the leaving group are relatively weak, and the carbon
has a substantial positive charge. However, the carbocation per se has no lifetime,
because bond rupture and formation occur concurrently. 14
13 J. P. Richard and W. P. Jencks, J. Am. Chem. Soc., 106, 1383 (1984).
14
B. L. Knier and W. P. Jencks, J. Am. Chem. Soc., 102, 6789 (1980); M. R. Skoog and W. P. Jencks, J.
Am. Chem. Soc., 106, 7597 (1984).