Page 418 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 418
399
SECTION 4.1
Mechanisms for
Nucleophilic Substitution
+
–
+
–
+
–
R X , R X , R X , Y –
Y – –
Y
intimate solvent- dissociated
ion pair separated ions
ion pair
R-X, Y –
R-Y, X –
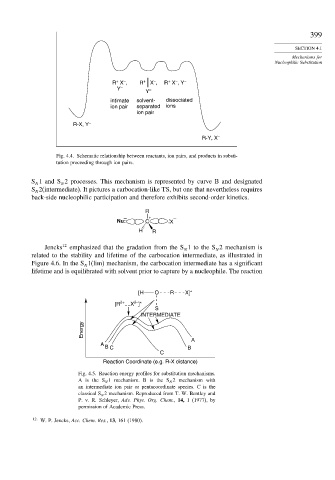
Fig. 4.4. Schematic relationship between reactants, ion pairs, and products in substi-
tution proceeding through ion pairs.
S 1 and S 2 processes. This mechanism is represented by curve B and designated
N
N
S 2(intermediate). It pictures a carbocation-like TS, but one that nevertheless requires
N
back-side nucleophilic participation and therefore exhibits second-order kinetics.
R
– + –
Nu: C X . .
H R
Jencks 12 emphasized that the gradation from the S 1 to the S 2 mechanism is
N
N
related to the stability and lifetime of the carbocation intermediate, as illustrated in
Figure 4.6. In the S 1(lim) mechanism, the carbocation intermediate has a significant
N
lifetime and is equilibrated with solvent prior to capture by a nucleophile. The reaction
[H O R X]*
δ+
δ–
[R ....X ]*
S
INTERMEDIATE
Energy
A
A
B C B
C
Reaction Coordinate (e.g. R-X distance)
Fig. 4.5. Reaction energy profiles for substitution mechanisms.
A is the S N 1 mechanism. B is the S N 2 mechanism with
an intermediate ion pair or pentacoordinate species. C is the
classical S N 2 mechanism. Reproduced from T. W. Bentley and
P. v. R. Schleyer, Adv. Phys. Org. Chem., 14, 1 (1977), by
permission of Academic Press.
12
W. P. Jencks, Acc. Chem. Res., 13, 161 (1980).