Page 415 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 415
396 proximity to one another. This species, called a contact ion pair (or intimate ion pair),
can proceed to a solvent-separated ion pair in which one or more solvent molecules
CHAPTER 4
are inserted between the carbocation and leaving group, but in which the ions are
Nucleophilic Substitution kept together by the electrostatic attraction. The “free carbocation,” characterized by
symmetrical solvation, is formed by diffusion from the anion, a process known as
dissociation.
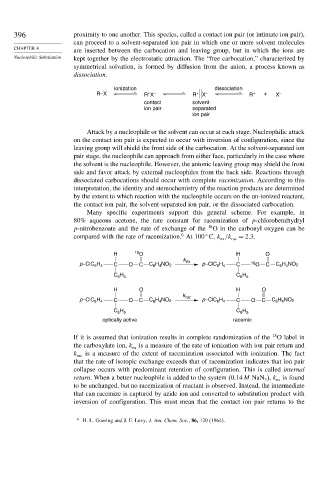
ionization dissociation
R–X R X R + X – R + + X –
+ –
contact solvent-
ion pair separated
ion pair
Attack by a nucleophile or the solvent can occur at each stage. Nucleophilic attack
on the contact ion pair is expected to occur with inversion of configuration, since the
leaving group will shield the front side of the carbocation. At the solvent-separated ion
pair stage, the nucleophile can approach from either face, particularly in the case where
the solvent is the nucleophile. However, the anionic leaving group may shield the front
side and favor attack by external nucleophiles from the back side. Reactions through
dissociated carbocations should occur with complete racemization. According to this
interpretation, the identity and stereochemistry of the reaction products are determined
by the extent to which reaction with the nucleophile occurs on the un-ionized reactant,
the contact ion pair, the solvent-separated ion pair, or the dissociated carbocation.
Many specific experiments support this general scheme. For example, in
80% aqueous acetone, the rate constant for racemization of p-chlorobenzhydryl
p-nitrobenzoate and the rate of exchange of the 18 O in the carbonyl oxygen can be
6
compared with the rate of racemization. At 100 C, k /k rac = 2 3.
ex
H 18 O H O
k
p–ClC H C O C C H NO 2 ex p–ClC H C 18 O C C H NO 2
6 4
6 4
6 4
6 4
H
C H C 6 5
6 5
H O H O
k rac
p–ClC H C O C C H NO 2 p–ClC H C O C C H NO 2
6 4
6 4
6 4
6 4
C H C H
6 5
6 5
optically active racemic
If it is assumed that ionization results in complete randomization of the 18 O label in
the carboxylate ion, k is a measure of the rate of ionization with ion pair return and
ex
k rac is a measure of the extent of racemization associated with ionization. The fact
that the rate of isotopic exchange exceeds that of racemization indicates that ion pair
collapse occurs with predominant retention of configuration. This is called internal
return. When a better nucleophile is added to the system (0 14M NaN ), k is found
3 ex
to be unchanged, but no racemization of reactant is observed. Instead, the intermediate
that can racemize is captured by azide ion and converted to substitution product with
inversion of configuration. This must mean that the contact ion pair returns to the
6
H. L. Goering and J. F. Levy, J. Am. Chem. Soc., 86, 120 (1964).