Page 411 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 411
392 As the rate-determining step is endothermic with a late TS, application of Hammond’s
postulate (Section 3.3.2.2) indicates that the TS should resemble the product of the
CHAPTER 4 first step, the carbocation intermediate. Ionization is facilitated by factors that lower
Nucleophilic Substitution the energy of the carbocation or raise the energy of the reactant. The rate of ionization
depends primarily on reactant structure, including the identity of the leaving group, and
the solvent’s ionizing power. The most important electronic effects are stabilization
of the carbocation by electron release, the ability of the leaving group to accept the
electron pair from the covalent bond that is broken, and the capacity of the solvent to
stabilize the charge separation that develops in the TS. Steric effects are also significant
because of the change in coordination that occurs on ionization. The substituents
are spread apart as ionization proceeds, so steric compression in the reactant favors
ionization. On the other hand, geometrical constraints that preclude planarity of the
carbocation are unfavorable and increase the energy required for ionization.
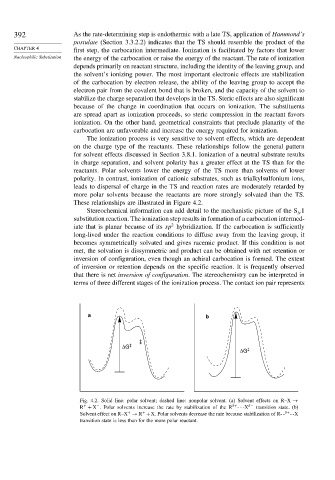
The ionization process is very sensitive to solvent effects, which are dependent
on the charge type of the reactants. These relationships follow the general pattern
for solvent effects discussed in Section 3.8.1. Ionization of a neutral substrate results
in charge separation, and solvent polarity has a greater effect at the TS than for the
reactants. Polar solvents lower the energy of the TS more than solvents of lower
polarity. In contrast, ionization of cationic substrates, such as trialkylsulfonium ions,
leads to dispersal of charge in the TS and reaction rates are moderately retarded by
more polar solvents because the reactants are more strongly solvated than the TS.
These relationships are illustrated in Figure 4.2.
Stereochemical information can add detail to the mechanistic picture of the S 1
N
substitution reaction. The ionization step results in formation of a carbocation intermed-
2
iate that is planar because of its sp hybridization. If the carbocation is sufficiently
long-lived under the reaction conditions to diffuse away from the leaving group, it
becomes symmetrically solvated and gives racemic product. If this condition is not
met, the solvation is dissymmetric and product can be obtained with net retention or
inversion of configuration, even though an achiral carbocation is formed. The extent
of inversion or retention depends on the specific reaction. It is frequently observed
that there is net inversion of configuration. The stereochemistry can be interpreted in
terms of three different stages of the ionization process. The contact ion pair represents
a b
‡
ΔG ‡
ΔG ‡
Fig. 4.2. Solid line: polar solvent; dashed line: nonpolar solvent. (a) Solvent effects on R–X →
R + X . Polar solvents increase the rate by stabilization of the R ---X − transition state. (b)
+
+
−
Solvent effect on R–X → R +X. Polar solvents decrease the rate because stabilization of R- - --X
+
+
+
transition state is less than for the more polar reactant.