Page 410 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 410
order to develop an understanding of the mechanisms of such reactions, we begin by 391
reviewing the limiting cases as defined by Hughes and Ingold, namely the ionization
mechanism (S 1, substitution-nucleophilic-unimolecular) and the direct displacement SECTION 4.1
N
mechanism (S 2, substitution-nucleophilic-bimolecular). We will find that in addition Mechanisms for
N Nucleophilic Substitution
to these limiting cases, there are related mechanisms that have aspects of both ionization
and direct displacement.
4.1.1. Substitution by the Ionization S 1 Mechanism
N
The ionization mechanism for nucleophilic substitution proceeds by rate-
determining heterolytic dissociation of the reactant to a tricoordinate carbocation 2
and the leaving group. This dissociation is followed by rapid combination of the
electrophilic carbocation with a Lewis base (nucleophile) present in the medium. A
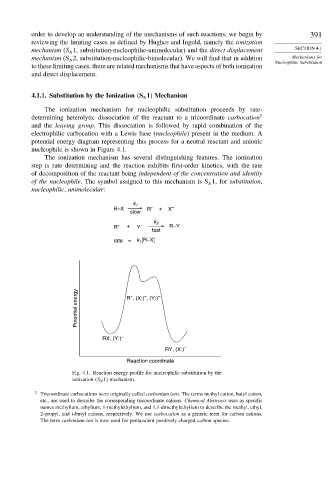
potential energy diagram representing this process for a neutral reactant and anionic
nucleophile is shown in Figure 4.1.
The ionization mechanism has several distinguishing features. The ionization
step is rate determining and the reaction exhibits first-order kinetics, with the rate
of decomposition of the reactant being independent of the concentration and identity
of the nucleophile. The symbol assigned to this mechanism is S 1, for substitution,
N
nucleophilic, unimolecular:
k 1
R–X R + + X –
slow
k 2
R + + Y – R–Y
fast
rate = k 1 [R–X]
Potential energy R , (X:) , (Y:) –
–
+
RX, (Y:) –
RY, (X:) –
Reaction coordinate
Fig. 4.1. Reaction energy profile for nucleophilic substitution by the
ionization S N 1 mechanism.
2
Tricoordinate carbocations were originally called carbonium ions. The terms methyl cation, butyl cation,
etc., are used to describe the corresponding tricoordinate cations. Chemical Abstracts uses as specific
names methylium, ethylium, 1-methylethylium, and 1,1-dimethylethylium to describe the methyl, ethyl,
2-propyl, and t-butyl cations, respectively. We use carbocation as a generic term for carbon cations.
The term carbonium ion is now used for pentavalent positively charged carbon species.