Page 407 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 407
388 (Continued)
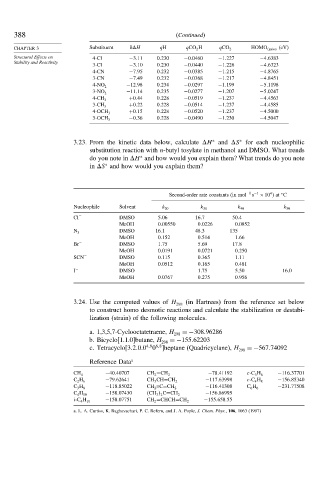
CHAPTER 3 Substituent H qH qCO 2 H qCO 2 − HOMO anion (eV)
Structural Effects on 4-Cl −3 11 0 230 −0 0460 −1 227 −4 6383
Stability and Reactivity
3-Cl −3 10 0 230 −0 0440 −1 226 −4 6323
4-CN −7 95 0 232 −0 0385 −1 215 −4 8765
3-CN −7 49 0 232 −0 0368 −1 217 −4 8451
−12 98 0 234 −0 0297 −1 199 −5 1198
4-NO 2
−11 14 0 235 −0 0277 −1 207 −5 0247
3-NO 2
+0 44 0 228 −0 0519 −1 237 −4 4563
4-CH 3
+0 22 0 228 −0 0514 −1 237 −4 4585
3-CH 3
+0 15 0 228 −0 0520 −1 237 −4 5000
4-OCH 3
−0 36 0 228 −0 0490 −1 230 −4 5047
3-OCH 3
3.23. From the kinetic data below, calculate H and S for each nucleophilic
∗
∗
substitution reaction with n-butyl tosylate in methanol and DMSO. What trends
∗
do you note in H and how would you explain them? What trends do you note
∗
in S and how would you explain them?
4
s
Second-order rate constants (in mol −1 −1 ×10 )at C
Nucleophile Solvent k 20 k 30 k 40 k 50
Cl − DMSO 5 06 16 7 50 4
MeOH 0 00550 0 0226 0 0852
− DMSO 16 1 48 3 135
N 3
MeOH 0 152 0 514 1 66
Br − DMSO 1 75 5 69 17 8
MeOH 0 0191 0 0721 0 250
SCN − DMSO 0 115 0 365 1 11
MeOH 0 0512 0 165 0 481
I − DMSO 1 75 5 50 16.0
MeOH 0 0767 0 275 0 956
3.24. Use the computed values of H 298 (in Hartrees) from the reference set below
to construct homo desmotic reactions and calculate the stabilization or destabi-
lization (strain) of the following molecules.
a. 1,3,5,7-Cyclooctatetraene, H 298 =−308 96286
b. Bicyclo[1.1.0]butane, H 298 =−155 62203
4 9 6 8
c. Tetracyclo 3 2 0 0 0 heptane (Quadricyclane), H 298 =−567 74092
Reference Data a
−40 40707 −78 41192 −116 37701
CH 4 CH 2 =CH 2 c-C 3 H 6
−79 62641 −117 63998 −156 85340
C 2 H 6 CH 3 CH=CH 2 c-C 4 H 8
−118 85022 −116 41308 −231 77508
C 3 H 8 CH 2 =C=CH 2 C 6 H 6
−158 07430 −156 86995
C 4 H 10 CH 3 2 C=CH 2
−158 07751 −155 658 55
i-C 4 H 10 CH 2 =CHCH=CH 2
a. L. A. Curtiss, K. Raghavachari, P. C. Refern, and J. A. Pople, J. Chem. Phys., 106, 1063 (1997)