Page 428 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 428
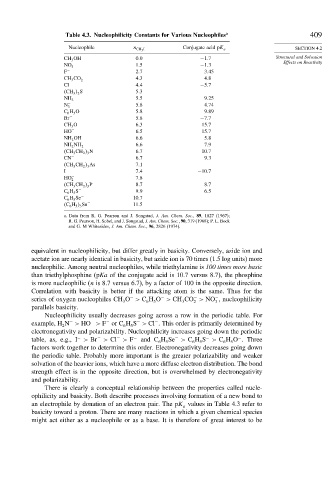
Table 4.3. Nucleophilicity Constants for Various Nucleophiles a 409
Nucleophile n CH 3 I Conjugate acid pK a SECTION 4.2
CH 3 OH 0 0 −1 7 Structural and Solvation
Effects on Reactivity
NO − 3 1 5 −1 3
F − 2 7 3 45
CH 3 CO − 2 4 3 4 8
Cl − 4 4 −5 7
CH 3 2 S 5 3
5 5 9 25
NH 3
N − 3 5 8 4 74
C 6 H 5 O − 5 8 9 89
Br − 5 8 −7 7
CH 3 O − 6 3 15 7
HO − 6 5 15 7
NH 2 OH 6 6 5 8
6 6 7 9
NH 2 NH 2
CH 3 CH 2 3 N 6 7 10 7
CN − 6 7 9 3
CH 3 CH 2 3 As 7 1
I − 7 4 −10 7
HO − 7 8
2
CH 3 CH 2 3 P 8 7 8 7
C 6 H 5 S − 9 9 6 5
C 6 H 5 Se − 10 7
C 6 H 5 3 Sn − 11 5
a. Data from R. G. Pearson and J. Songstad, J. Am. Chem. Soc., 89, 1827 (1967);
R. G. Pearson, H. Sobel, and J. Songstad, J. Am. Chem. Soc., 90, 319 (1968); P. L. Bock
and G. M Whitesides, J. Am. Chem. Soc., 96, 2826 (1974).
equivalent in nucleophilicity, but differ greatly in basicity. Conversely, azide ion and
acetate ion are nearly identical in basicity, but azide ion is 70 times (1.5 log units) more
nucleophilic. Among neutral nucleophiles, while triethylamine is 100 times more basic
than triethylphosphine (pKa of the conjugate acid is 10.7 versus 8.7), the phosphine
is more nucleophilic (n is 8.7 versus 6.7), by a factor of 100 in the opposite direction.
Correlation with basicity is better if the attacking atom is the same. Thus for the
−
−
−
−
series of oxygen nucleophiles CH O > C H O > CH CO > NO , nucleophilicity
5
6
3
3
2
3
parallels basicity.
Nucleophilicity usually decreases going across a row in the periodic table. For
−
example, H N > HO > F or C H S > Cl . This order is primarily determined by
−
−
−
−
6
2
5
electronegativity and polarizability. Nucleophilicity increases going down the periodic
−
−
−
table, as, e.g., I > Br > Cl > F − and C H Se > C H S > C H O . Three
−
−
−
6 5 6 5 6 5
factors work together to determine this order. Electronegativity decreases going down
the periodic table. Probably more important is the greater polarizability and weaker
solvation of the heavier ions, which have a more diffuse electron distribution. The bond
strength effect is in the opposite direction, but is overwhelmed by electronegativity
and polarizability.
There is clearly a conceptual relationship between the properties called nucle-
ophilicity and basicity. Both describe processes involving formation of a new bond to
an electrophile by donation of an electron pair. The pK values in Table 4.3 refer to
a
basicity toward a proton. There are many reactions in which a given chemical species
might act either as a nucleophile or as a base. It is therefore of great interest to be