Page 432 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 432
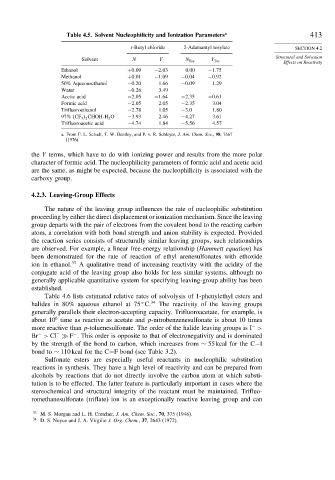
Table 4.5. Solvent Nucleophilicity and Ionization Parameters a 413
t-Butyl chloride 2-Adamantyl tosylate SECTION 4.2
Structural and Solvation
Solvent N Y N Tos Y Tos
Effects on Reactivity
Ethanol +0 09 −2 03 0.00 −1 75
Methanol +0 01 −1 09 −0 04 −0 92
50% Aqueousethanol −0 20 1.66 −0 09 1.29
Water −0 26 3.49
Acetic acid −2 05 −1 64 −2 35 −0 61
Formic acid −2 05 2.05 −2 35 3.04
Trifluoroethanol −2 78 1.05 −3 0 1.80
97% CF 3 2 CHOH-H 2 O −3 93 2.46 −4 27 3.61
Trifluoroacetic acid −4 74 1.84 −5 56 4.57
a. From F. L. Schadt, T. W. Bentley, and P. v. R. Schleyer, J. Am. Chem. Soc., 98, 7667
(1976).
the Y terms, which have to do with ionizing power and results from the more polar
character of formic acid. The nucleophilicity parameters of formic acid and acetic acid
are the same, as might be expected, because the nucleophilicity is associated with the
carboxy group.
4.2.3. Leaving-Group Effects
The nature of the leaving group influences the rate of nucleophilic substitution
proceeding by either the direct displacement or ionization mechanism. Since the leaving
group departs with the pair of electrons from the covalent bond to the reacting carbon
atom, a correlation with both bond strength and anion stability is expected. Provided
the reaction series consists of structurally similar leaving groups, such relationships
are observed. For example, a linear free-energy relationship (Hammett equation) has
been demonstrated for the rate of reaction of ethyl arenesulfonates with ethoxide
ion in ethanol. 33 A qualitative trend of increasing reactivity with the acidity of the
conjugate acid of the leaving group also holds for less similar systems, although no
generally applicable quantitative system for specifying leaving-group ability has been
established.
Table 4.6 lists estimated relative rates of solvolysis of 1-phenylethyl esters and
halides in 80% aqueous ethanol at 75 C. 34 The reactivity of the leaving groups
generally parallels their electron-accepting capacity. Trifluoroacetate, for example, is
6
about 10 time as reactive as acetate and p-nitrobenzenesulfonate is about 10 times
more reactive than p-toluenesulfonate. The order of the halide leaving groups is I >
−
−
Br > Cl F . This order is opposite to that of electronegativity and is dominated
−
−
by the strength of the bond to carbon, which increases from ∼ 55kcal for the C−I
bond to ∼ 110kcal for the C−F bond (see Table 3.2).
Sulfonate esters are especially useful reactants in nucleophilic substitution
reactions in synthesis. They have a high level of reactivity and can be prepared from
alcohols by reactions that do not directly involve the carbon atom at which substi-
tution is to be effected. The latter feature is particularly important in cases where the
stereochemical and structural integrity of the reactant must be maintained. Trifluo-
romethanesulfonate (triflate) ion is an exceptionally reactive leaving group and can
33 M. S. Morgan and L. H. Cretcher, J. Am. Chem. Soc., 70, 375 (1948).
34
D. S. Noyce and J. A. Virgilio J. Org. Chem., 37, 2643 (1972).