Page 435 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 435
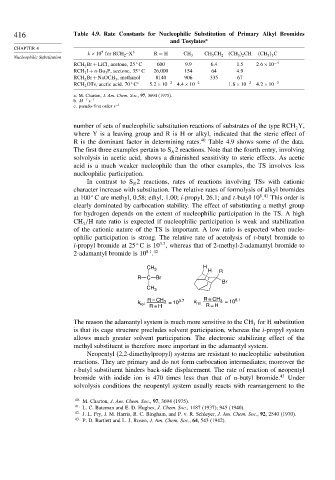
416 Table 4.9. Rate Constants for Nucleophilic Substitution of Primary Alkyl Bromides
and Tosylates a
CHAPTER 4
5
k×10 for RCH 2 –X b R = H CH 3 CH 3 CH 2 CH 3 2 CH CH 3 3 C
Nucleophilic Substitution
RCH 2 Br +LiCl, acetone, 25 C 600 9.9 6.4 1.5 2 6×10 −4
RCH 2 I +n-Bu 3 P, acetone, 35 C 26,000 154 64 4.9
RCH 2 Br +NaOCH 3 , methanol 8140 906 335 67
RCH 2 OTs, acetic acid. 70 C c 5 2×10 −2 4 4×10 −2 1 8×10 −2 4 2×10 −3
a. M. Charton, J. Am. Chem. Soc., 97, 3694 (1975).
b. M −1 −1
s
c. pseudo-first order s −1
number of sets of nucleophilic substitution reactions of substrates of the type RCH Y,
2
where Y is a leaving group and R is H or alkyl, indicated that the steric effect of
R is the dominant factor in determining rates. 40 Table 4.9 shows some of the data.
The first three examples pertain to S 2 reactions. Note that the fourth entry, involving
N
solvolysis in acetic acid, shows a diminished sensitivity to steric effects. As acetic
acid is a much weaker nucleophile than the other examples, the TS involves less
nucleophilic participation.
In contrast to S 2 reactions, rates of reactions involving TSs with cationic
N
character increase with substitution. The relative rates of formolysis of alkyl bromides
8 41
at 100 C are methyl, 0.58; ethyl, 1.00; i-propyl, 26.1; and t-butyl 10 . This order is
clearly dominated by carbocation stability. The effect of substituting a methyl group
for hydrogen depends on the extent of nucleophilic participation in the TS. A high
CH /H rate ratio is expected if nucleophilic participation is weak and stabilization
3
of the cationic nature of the TS is important. A low ratio is expected when nucle-
ophilic participation is strong. The relative rate of acetolysis of t-butyl bromide to
3 7
i-propyl bromide at 25 Cis10 , whereas that of 2-methyl-2-adamantyl bromide to
8 1 42
2-adamantyl bromide is 10 .
CH 3 H H R
R C Br
Br
CH 3
k rel R = CH 3 = 10 3.7 k rel R = CH 3 = 10 8.1
R = H R = H
The reason the adamantyl system is much more sensitive to the CH for H substitution
3
is that its cage structure precludes solvent participation, whereas the i-propyl system
allows much greater solvent participation. The electronic stabilizing effect of the
methyl substituent is therefore more important in the adamantyl system.
Neopentyl (2,2-dimethylpropyl) systems are resistant to nucleophilic substitution
reactions. They are primary and do not form carbocation intermediates; moreover the
t-butyl substituent hinders back-side displacement. The rate of reaction of neopentyl
bromide with iodide ion is 470 times less than that of n-butyl bromide. 43 Under
solvolysis conditions the neopentyl system usually reacts with rearrangement to the
40
M. Charton, J. Am. Chem. Soc., 97, 3694 (1975).
41
L. C. Bateman and E. D. Hughes, J. Chem. Soc., 1187 (1937); 945 (1940).
42 J. L. Fry, J. M. Harris, R. C. Bingham, and P. v. R. Schleyer, J. Am. Chem. Soc., 92, 2540 (1970).
43
P. D. Bartlett and L. J. Rosen, J. Am. Chem. Soc., 64, 543 (1942).