Page 439 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 439
420 energetically favorable. The cation that is formed by participation is stabilized by
both acetoxy oxygen atoms and is far more stable than a secondary carbocation. The
CHAPTER 4 resulting acetoxonium ion intermediate is subsequently opened by nucleophilic attack
Nucleophilic Substitution with inversion at either of the two equivalent carbons, leading to the observed trans
product. 54
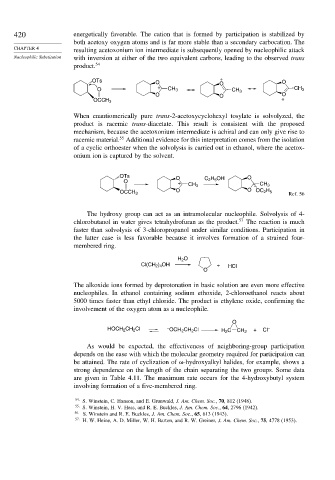
OTs O + O
O
O + CH 3 CH 3 CH 3
O O O
OCCH 3 +
When enantiomerically pure trans-2-acetoxycyclohexyl tosylate is solvolyzed, the
product is racemic trans-diacetate. This result is consistent with the proposed
mechanism, because the acetoxonium intermediate is achiral and can only give rise to
55
racemic material. Additional evidence for this interpretation comes from the isolation
of a cyclic orthoester when the solvolysis is carried out in ethanol, where the acetox-
onium ion is captured by the solvent.
OTs O C 2 H 5 OH O
O +
CH 3 CH 3
O
Ref. 56
OCCH 3 O OC 2 H 5
The hydroxy group can act as an intramolecular nucleophile. Solvolysis of 4-
chlorobutanol in water gives tetrahydrofuran as the product. 57 The reaction is much
faster than solvolysis of 3-chloropropanol under similar conditions. Participation in
the latter case is less favorable because it involves formation of a strained four-
membered ring.
H 2 O
Cl(CH 2 ) 4 OH + HCl
O
The alkoxide ions formed by deprotonation in basic solution are even more effective
nucleophiles. In ethanol containing sodium ethoxide, 2-chloroethanol reacts about
5000 times faster than ethyl chloride. The product is ethylene oxide, confirming the
involvement of the oxygen atom as a nucleophile.
O
HOCH 2 CH 2 Cl – OCH 2 CH 2 Cl H 2 C CH 2 + Cl –
As would be expected, the effectiveness of neighboring-group participation
depends on the ease with which the molecular geometry required for participation can
be attained. The rate of cyclization of -hydroxyalkyl halides, for example, shows a
strong dependence on the length of the chain separating the two groups. Some data
are given in Table 4.11. The maximum rate occurs for the 4-hydroxybutyl system
involving formation of a five-membered ring.
54
S. Winstein, C. Hanson, and E. Grunwald, J. Am. Chem. Soc., 70, 812 (1948).
55 S. Winstein, H. V. Hess, and R. E. Buckles, J. Am. Chem. Soc., 64, 2796 (1942).
56 S. Winstein and R. E. Buckles, J. Am. Chem. Soc., 65, 613 (1943).
57
H. W. Heine, A. D. Miller, W. H. Barton, and R. W. Greiner, J. Am. Chem. Soc., 75, 4778 (1953).