Page 443 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 443
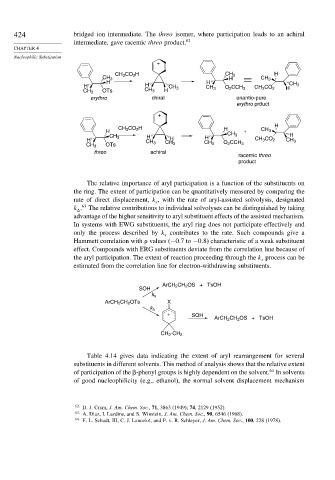
424 bridged ion intermediate. The threo isomer, where participation leads to an achiral
intermediate, gave racemic threo product. 62
CHAPTER 4
Nucleophilic Substitution
+
CH 3 CO 2 H CH 3 H
CH 3 H CH 3
H H
H H CH 3 CH 3 O 2 CCH 3 CH 3 CO 2 H CH 3
CH 3 OTs CH 3 H
erythro chiral enantio-pure
erythro prduct
+
H
CH 3 CO 2 H H
H + CH 3
CH 3 H H CH 3 H
H CH 3 H CH 3 CO 2 CH 3
CH 3 OTs CH 3 CH 3 O 2 CCH 3
threo achiral
racemic threo
product
The relative importance of aryl participation is a function of the substituents on
the ring. The extent of participation can be quantitatively measured by comparing the
rate of direct displacement, k , with the rate of aryl-assisted solvolysis, designated
s
63
k . The relative contributions to individual solvolyses can be distinguished by taking
advantage of the higher sensitivity to aryl substituent effects of the assisted mechanism.
In systems with EWG substituents, the aryl ring does not participate effectively and
only the process described by k contributes to the rate. Such compounds give a
s
Hammett correlation with
values (−0 7to −0 8) characteristic of a weak substituent
effect. Compounds with ERG substituents deviate from the correlation line because of
the aryl participation. The extent of reaction proceeding through the k process can be
s
estimated from the correlation line for electron-withdrawing substituents.
ArCH 2 CH 2 OS + TsOH
SOH
k s
ArCH 2 CH 2 OTs X
k Δ
+ SOH
ArCH 2 CH 2 OS + TsOH
CH 2 -CH 2
Table 4.14 gives data indicating the extent of aryl rearrangement for several
substituents in different solvents. This method of analysis shows that the relative extent
64
of participation of the -phenyl groups is highly dependent on the solvent. In solvents
of good nucleophilicity (e.g., ethanol), the normal solvent displacement mechanism
62 D. J. Cram, J. Am. Chem. Soc., 71, 3863 (1949); 74, 2129 (1952).
63 A. Diaz, I. Lazdins, and S. Winstein, J. Am. Chem. Soc., 90, 6546 (1968).
64
F. L. Schadt, III, C. J. Lancelot, and P. v. R. Schleyer, J. Am. Chem. Soc., 100, 228 (1978).