Page 441 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 441
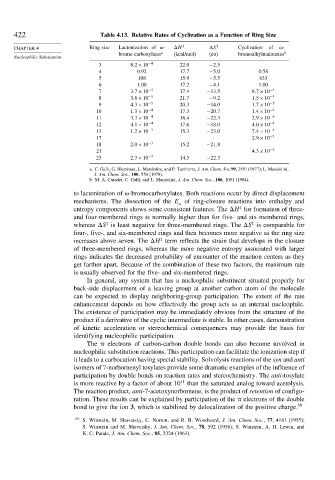
422 Table 4.13. Relative Rates of Cyclization as a Function of Ring Size
CHAPTER 4 Ring size Lactonization of - H ‡ S ‡ Cyclization of -
bromo carboxylates a (kcal/mol) (eu) bromoalkylmalonates b
Nucleophilic Substitution
3 8 2×10 −4 22 0 −2 5
4 0.92 17 7 −5 0 0.58
5 108 15 9 −5 5 833
6 1.00 17 2 −4 1 1.00
7 3 7×10 −3 17 4 −13 5 8 7×10 −3
8 3 8×10 −5 21 7 −9 2 1 5×10 −4
9 4 3×10 −5 20 3 −14 0 1 7×10 −5
10 1 3×10 −4 17 3 −20 7 1 4×10 −6
11 3 3×10 −4 16 4 −22 3 2 9×10 −6
12 4 1×10 −4 17 6 −18 0 4 0×10 −4
13 1 2×10 −3 15 3 −23 0 7 4×10 −4
17 2 9×10 −3
18 2 0×10 −3 15 2 −21 8
21 4 3×10 −3
23 2 3×10 −3 14 5 −22 3
a. C. Galli, G. Illuminati, L. Mandolini, and P. Tamborra, J. Am. Chem. Soc.99, 2591 (1977); L. Mandolini,
J. Am. Chem. Soc., 100, 550 (1978).
b. M. A. Casadei, C. Galli, and L. Mandolini, J. Am. Chem. Soc., 106, 1051 (1984).
to lactonization of -bromocarboxylates. Both reactions occur by direct displacement
mechanisms. The dissection of the E of ring-closure reactions into enthalpy and
a
‡
entropy components shows some consistent features. The H for formation of three-
and four-membered rings is normally higher than for five- and six-membered rings,
‡
‡
whereas S is least negative for three-membered rings. The S is comparable for
four-, five-, and six-membered rings and then becomes more negative as the ring size
‡
increases above seven. The H term reflects the strain that develops in the closure
of three-membered rings, whereas the more negative entropy associated with larger
rings indicates the decreased probability of encounter of the reaction centers as they
get farther apart. Because of the combination of these two factors, the maximum rate
is usually observed for the five- and six-membered rings.
In general, any system that has a nucleophilic substituent situated properly for
back-side displacement of a leaving group at another carbon atom of the molecule
can be expected to display neighboring-group participation. The extent of the rate
enhancement depends on how effectively the group acts as an internal nucleophile.
The existence of participation may be immediately obvious from the structure of the
product if a derivative of the cyclic intermediate is stable. In other cases, demonstration
of kinetic acceleration or stereochemical consequences may provide the basis for
identifying nucleophilic participation.
The electrons of carbon-carbon double bonds can also become involved in
nucleophilic substitution reactions. This participation can facilitate the ionization step if
it leads to a carbocation having special stability. Solvolysis reactions of the syn and anti
isomers of 7-norbornenyl tosylates provide some dramatic examples of the influence of
participation by double bonds on reaction rates and stereochemistry. The anti-tosylate
is more reactive by a factor of about 10 11 than the saturated analog toward acetolysis.
The reaction product, anti-7-acetoxynorbornene, is the product of retention of configu-
ration. These results can be explained by participation of the electrons of the double
bond to give the ion 3, which is stabilized by delocalization of the positive charge. 59
59 S. Winstein, M. Shavatsky, C. Norton, and R. B. Woodward, J. Am. Chem. Soc., 77, 4183 (1955);
S. Winstein and M. Shavatsky, J. Am. Chem. Soc., 78, 592 (1956); S. Winstein, A. H. Lewin, and
K. C. Pande, J. Am. Chem. Soc., 85, 2324 (1963).