Page 444 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 444
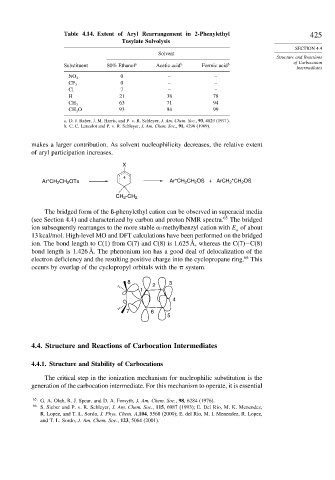
Table 4.14. Extent of Aryl Rearrangement in 2-Phenylethyl 425
Tosylate Solvolysis
SECTION 4.4
Solvent
Structure and Reactions
of Carbocation
Substituent 80% Ethanol a Acetic acid b Formic acid b
Intermediates
0 – –
NO 2
0 – –
CF 3
Cl 7 – –
H 21 38 78
63 71 94
CH 3
CH 3 O 93 94 99
a. D. J. Raber, J. M. Harris, and P. v. R. Schleyer, J. Am. Chem. Soc., 93, 4829 (1971).
b. C. C. Lancelot and P. v. R. Schleyer, J. Am. Chem. Soc., 91, 4296 (1969).
makes a larger contribution. As solvent nucleophilicity decreases, the relative extent
of aryl participation increases.
X
+
Ar*CH 2 CH 2 OTs Ar*CH 2 CH 2 OS + ArCH 2 *CH 2 OS
CH 2 -CH 2
The bridged form of the ß-phenylethyl cation can be observed in superacid media
65
(see Section 4.4) and characterized by carbon and proton NMR spectra. The bridged
ion subsequently rearranges to the more stable
-methylbenzyl cation with E of about
a
13 kcal/mol. High-level MO and DFT calculations have been performed on the bridged
ion. The bond length to C(1) from C(7) and C(8) is 1.625 Å, whereas the C(7)−C(8)
bond length is 1.426 Å. The phenonium ion has a good deal of delocalization of the
66
electron deficiency and the resulting positive charge into the cyclopropane ring. This
occurs by overlap of the cyclopropyl orbitals with the system.
8 3
2
1
4
7 6
5
4.4. Structure and Reactions of Carbocation Intermediates
4.4.1. Structure and Stability of Carbocations
The critical step in the ionization mechanism for nucleophilic substitution is the
generation of the carbocation intermediate. For this mechanism to operate, it is essential
65 G. A. Olah, R. J. Spear, and D. A. Forsyth, J. Am. Chem. Soc., 98, 6284 (1976).
66
S. Sieber and P. v. R. Schleyer, J. Am. Chem. Soc., 115, 6987 (1993); E. Del Rio, M. K. Menendez,
R. Lopez, and T. L. Sordo, J. Phys. Chem. A,104, 5568 (2000); E. del Rio, M. I. Menendez, R. Lopez,
and T. L. Sordo, J. Am. Chem. Soc., 123, 5064 (2001).