Page 442 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 442
OTs δ − OTs O 2 CCH 3 423
δ + + CH 3 CO 2 H SECTION 4.3
Neighboring-Group
Participation
3
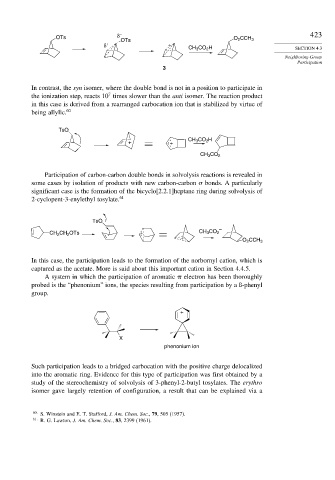
In contrast, the syn isomer, where the double bond is not in a position to participate in
7
the ionization step, reacts 10 times slower than the anti isomer. The reaction product
in this case is derived from a rearranged carbocation ion that is stabilized by virtue of
being allylic. 60
TsO
CH 3 CO 2 H
+ +
CH 3 CO 2
Participation of carbon-carbon double bonds in solvolysis reactions is revealed in
some cases by isolation of products with new carbon-carbon bonds. A particularly
significant case is the formation of the bicyclo[2.2.1]heptane ring during solvolysis of
2-cyclopent-3-enylethyl tosylate. 61
TsO
–
CH CH OTs + CH 3 CO 2
2
2
+
O 2 CCH 3
In this case, the participation leads to the formation of the norbornyl cation, which is
captured as the acetate. More is said about this important cation in Section 4.4.5.
A system in which the participation of aromatic electron has been thoroughly
probed is the “phenonium” ions, the species resulting from participation by a ß-phenyl
group.
+
X
phenonium ion
Such participation leads to a bridged carbocation with the positive charge delocalized
into the aromatic ring. Evidence for this type of participation was first obtained by a
study of the stereochemistry of solvolysis of 3-phenyl-2-butyl tosylates. The erythro
isomer gave largely retention of configuration, a result that can be explained via a
60 S. Winstein and E. T. Stafford, J. Am. Chem. Soc., 79, 505 (1957).
61
R. G. Lawton, J. Am. Chem. Soc., 83, 2399 (1961).