Page 438 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 438
withdrawal is more stabilizing. Thus substituents such as carbonyl have their greatest 419
effect on reactions with strong nucleophiles. Adjacent alkoxy substituents act as
donors and can stabilize S 2 TSs that are cationic in character. Vinyl and phenyl SECTION 4.3
N
groups can stabilize either type of TS, and allyl and benzyl systems show enhanced Neighboring-Group
Participation
reactivity toward both strong and weak nucleophiles. 51
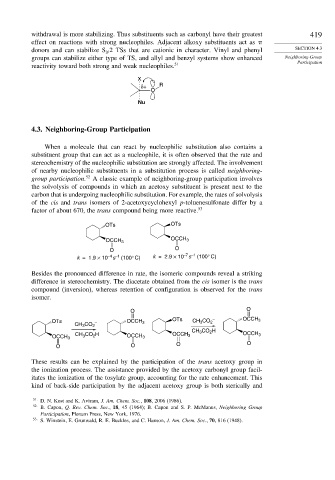
X
δ+ R
O
Nu
4.3. Neighboring-Group Participation
When a molecule that can react by nucleophilic substitution also contains a
substituent group that can act as a nucleophile, it is often observed that the rate and
stereochemistry of the nucleophilic substitution are strongly affected. The involvement
of nearby nucleophilic substituents in a substitution process is called neighboring-
group participation. 52 A classic example of neighboring-group participation involves
the solvolysis of compounds in which an acetoxy substituent is present next to the
carbon that is undergoing nucleophilic substitution. For example, the rates of solvolysis
of the cis and trans isomers of 2-acetoxycyclohexyl p-toluenesulfonate differ by a
factor of about 670, the trans compound being more reactive. 53
OTs OTs
OCCH 3
OCCH 3
O O
–7 –1
–4 –1
k = 1.9 × 10 s (100° C) k = 2.9 × 10 s (100° C)
Besides the pronounced difference in rate, the isomeric compounds reveal a striking
difference in stereochemistry. The diacetate obtained from the cis isomer is the trans
compound (inversion), whereas retention of configuration is observed for the trans
isomer.
O O
OTs – OCCH 3 OTs CH 3 CO 2 – OCCH 3
CH 3 CO 2
CH 3 CO 2 H
CH CO H OCCH 3 OCCH 3
OCCH 3 3 2 OCCH 3
O O O O
These results can be explained by the participation of the trans acetoxy group in
the ionization process. The assistance provided by the acetoxy carbonyl group facil-
itates the ionization of the tosylate group, accounting for the rate enhancement. This
kind of back-side participation by the adjacent acetoxy group is both sterically and
51 D. N. Kost and K. Aviram, J. Am. Chem. Soc., 108, 2006 (1986).
52 B. Capon, Q. Rev. Chem. Soc., 18, 45 (1964); B. Capon and S. P. McManus, Neighboring Group
Participation, Plenum Press, New York, 1976.
53
S. Winstein, E. Grunwald, R. E. Buckles, and C. Hanson, J. Am. Chem. Soc., 70, 816 (1948).