Page 446 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 446
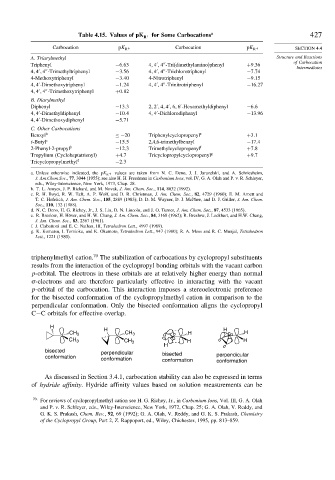
Table 4.15. Values of pK + for Some Carbocations a 427
R
Carbocation pK R + Carbocation pK R + SECTION 4.4
A. Triarylmethyl Structure and Reactions
of Carbocation
Triphenyl −6 63 4 4 4 -Tri(dimethylamino)phenyl +9 36
Intermediates
4 4 4 -Trimethyltriphenyl −3 56 4 4 4 -Trichlorotriphenyl −7 74
4-Methoxytriphenyl −3 40 4-Nitrotriphenyl −9 15
4 4 -Dimethoxytriphenyl −1 24 4 4 4 -Trinitrotriphenyl −16 27
4 4 4 -Trimethoxytriphenyl +0 82
B. Diarylmethyl
Diphenyl −13 3 2 2 4 4 6 6 -Hexamethyldiphenyl −6 6
4 4 -Dimethyldiphenyl −10 4 4 4 -Dichlorodiphenyl −13 96
4 4 -Dimethoxydiphenyl −5 71
C. Other Carbocations
Benzyl b ≤−20 Triphenylcyclopropenyl e +3 1
t-Butyl c −15 5 2,4,6-trimethylbenzyl −17 4
2-Phenyl-2-propyl b −12 3 Trimethylcyclopropenyl f +7 8
Tropylium (Cycloheptatrienyl) +4 7 Tricyclopropylcyclopropenyl g +9 7
Tricyclopropylmethyl d −2 3
a. Unless otherwise indicated, the pK R + values are taken from N. C. Deno, J. J. Jaruzelski, and A. Schriesheim,
J. Am.Chem.Soc., 77, 3044 (1955); see also H. H. Freedman in Carbonium Ions, vol. IV, G. A. Olah and P. v. R. Schleyer,
eds., Wiley-Interscience, New York, 1973, Chap. 28.
b. T. L. Amyes, J. P. Richard, and M. Novak, J. Am. Chem. Soc., 114, 8032 (1992).
c. R. H. Boyd, R. W. Taft, A. P. Wolf, and D. R. Christman, J. Am. Chem. Soc., 82, 4729 (1960); E. M. Arnett and
T. C. Hofelich, J Am. Chem. Soc., 105, 2889 (1983); D. D. M. Wayner, D. J. McPhee, and D. J. Griller, J. Am. Chem.
Soc., 110, 132 (1988).
d. N. C. Deno, H. G. Richey, Jr., J. S. Liu, D. N. Lincoln, and J. O. Turner, J. Am. Chem. Soc., 87, 4533 (1965).
e. R. Breslow, H. Höver, and H. W. Chang, J. Am. Chem. Soc., 84, 3168 (1962); R. Breslow, J. Lockhart, and H.W. Chang,
J. Am. Chem. Soc., 83, 2367 (1961).
f. J. Ciabattoni and E. C. Nathan, III, Tetrahedron Lett., 4997 (1969).
g. K. Komatsu, I. Tomioka, and K. Okamoto, Tetrahedron Lett., 947 (1980); R. A. Moss and R. C. Munjal, Tetrahedron
Lett., 1221 (1980).
70
triphenylmethyl cation. The stabilization of carbocations by cyclopropyl substituents
results from the interaction of the cyclopropyl bonding orbitals with the vacant carbon
p-orbital. The electrons in these orbitals are at relatively higher energy than normal
-electrons and are therefore particularly effective in interacting with the vacant
p-orbital of the carbocation. This interaction imposes a stereoelectronic preference
for the bisected conformation of the cyclopropylmethyl cation in comparison to the
perpendicular conformation. Only the bisected conformation aligns the cyclopropyl
C−C orbitals for effective overlap.
H
H H
CH 3 CH 3 H H
CH 3 H H
CH 3
H
bisected perpendicular
conformation conformation bisected perpendicular
conformation
conformation
As discussed in Section 3.4.1, carbocation stability can also be expressed in terms
of hydride affinity. Hydride affinity values based on solution measurements can be
70
For reviews of cyclopropylmethyl cation see H. G. Richey, Jr., in Carbonium Ions, Vol. III, G. A. Olah
and P. v. R. Schleyer, eds., Wiley-Interscience, New York, 1972, Chap. 25; G. A. Olah, V. Reddy, and
G. K. S. Prakash, Chem. Rev., 92, 69 (1992); G. A. Olah, V. Reddy, and G. K. S. Prakash, Chemistry
of the Cyclopropyl Group, Part 2, Z. Rappoport, ed., Wiley, Chichester, 1995, pp. 813–859.