Page 451 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 451
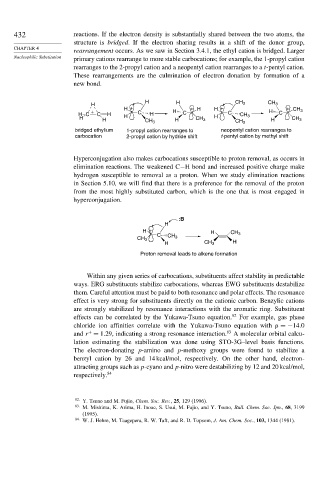
432 reactions. If the electron density is substantially shared between the two atoms, the
structure is bridged. If the electron sharing results in a shift of the donor group,
CHAPTER 4
rearrangement occurs. As we saw in Section 3.4.1, the ethyl cation is bridged. Larger
Nucleophilic Substitution
primary cations rearrange to more stable carbocations; for example, the 1-propyl cation
rearranges to the 2-propyl cation and a neopentyl cation rearranges to a t-pentyl cation.
These rearrangements are the culmination of electron donation by formation of a
new bond.
H H
H CH 3 CH 3
H + + H H + +
+ C H H CH 3
H C C H H H C C CH 3 C
H H CH 3 H CH 3 H CH 3 H CH 3
bridged ethylium 1-propyl cation rearranges to neopentyl cation rearranges to
carbocation 2-propyl cation by hydride shift t-pentyl cation by methyl shift
Hyperconjugation also makes carbocations susceptible to proton removal, as occurs in
elimination reactions. The weakened C−H bond and increased positive charge make
hydrogen susceptible to removal as a proton. When we study elimination reactions
in Section 5.10, we will find that there is a preference for the removal of the proton
from the most highly substituted carbon, which is the one that is most engaged in
hyperconjugation.
:B
H
H + H
C CH 3 CH 3
CH 3 H
H CH 3
Proton removal leads to alkene formation
Within any given series of carbocations, substituents affect stability in predictable
ways. ERG substituents stabilize carbocations, whereas EWG substituents destabilize
them. Careful attention must be paid to both resonance and polar effects. The resonance
effect is very strong for substituents directly on the cationic carbon. Benzylic cations
are strongly stabilized by resonance interactions with the aromatic ring. Substituent
effects can be correlated by the Yukawa-Tsuno equation. 82 For example, gas phase
chloride ion affinities correlate with the Yukawa-Tsuno equation with
=−14 0
and r = 1 29, indicating a strong resonance interaction. 83 A molecular orbital calcu-
+
lation estimating the stabilization was done using STO-3G–level basis functions.
The electron-donating p-amino and p-methoxy groups were found to stabilize a
benzyl cation by 26 and 14 kcal/mol, respectively. On the other hand, electron-
attracting groups such as p-cyano and p-nitro were destabilizing by 12 and 20 kcal/mol,
respectively. 84
82
Y. Tsuno and M. Fujio, Chem. Soc. Rev., 25, 129 (1996).
83 M. Mishima, K. Arima, H. Inoue, S. Usui, M. Fujio, and Y. Tsuno, Bull. Chem. Soc. Jpn., 68, 3199
(1995).
84
W. J. Hehre, M. Taagepera, R. W. Taft, and R. D. Topsom, J. Am. Chem. Soc., 103, 1344 (1981).