Page 456 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 456
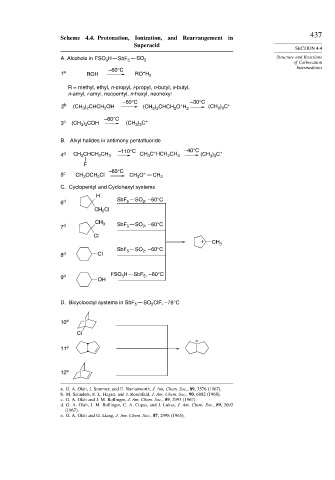
437
Scheme 4.4. Protonation, Ionization, and Rearrangement in
Superacid
SECTION 4.4
A. Alcohols in FSO H SbF 5 SO 2 Structure and Reactions
3
of Carbocation
Intermediates
–60°C
+
1 a ROH RO H 2
R = methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl,
n-amyl, i-amyl, neopentyl, n-hexyl, neohexyl
–60°C –30°C
+
) C
2 b (CH ) CHCH OH (CH ) CHCH O H 2 (CH 3 3 +
2
3 2
2
3 2
–60°C
) C
) COH
3 c (CH 3 3 (CH 3 3 +
B. Alkyl halides in antimony pentafluoride
–110°C + –40°C +
4 d CH 3 CHCH CH 3 CH C HCH CH 3 (CH ) C
2
2
3 3
3
F
–60°C
5 c CH OCH Cl CH O + CH 2
2
3
3
C. Cyclopentyl and Cyclohexyl systems
H
2
6 d SbF 5 SO , – 60°C
CH 2 Cl
CH 3 SbF SO , – 60°C
7 d 5 2
Cl
+ CH 3
SbF 5 SO , – 60°C
2
8 d Cl
5
3
9 d OH FSO H SbF , – 60°C
D. Bicyclooctyl systems in SbF 5 SO ClF, –78°C
2
10 e
Cl
+
11 e
12 e
a. G. A. Olah, J. Sommer, and E. Namanworth, J. Am. Chem. Soc., 89, 3576 (1967).
b. M. Saunders, F. L. Hagen, and J. Rosenfeld, J. Am. Chem. Soc., 90, 6882 (1968).
c. G. A. Olah and J. M. Bollinger, J. Am. Chem. Soc., 89, 2993 (1967).
d. G. A. Olah, J. M. Bollinger, C. A. Cupas, and J. Lukas, J. Am. Chem. Soc., 89, 2692
(1967).
e. G. A. Olah and G. Liang, J. Am. Chem. Soc., 87, 2998 (1965).