Page 461 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 461
+
442 There have been two extensive MO studies of the C H species. The most stable
4
9
structure is the t-butyl cation. At the MP4/6-311G ∗∗ level, the H-bridged structure A
CHAPTER 4
was the next most stable structure and it was 2.3 kcal more stable than the open 2-
Nucleophilic Substitution butyl cation D. 122 The methyl-bridged ion B is only slightly less stable. The structures
∗
were also calculated at the MP4/6-31G level of theory. 123 The energies relative to the
t-butyl cation are similar, although the methyl-bridged ion B is found to be slightly
+
more stable than the hydride-bridged ion A. Relative energies of C H cations are
4
9
shown below in kcal/mol.
CH 3 +
CH 3
H+ CH 3 CH 3
CH 3 CH 3
+
CH 3 CH 2 +
CH 3 CH 3 +
CH 3 CH 3 CH 3
B H C D E F
A +
MP/6-311G* 13.4 13.8 21.9 15.7 33.0 0.0
MP/6-31G** 12.8 12.0 20.5 32.6 0.0
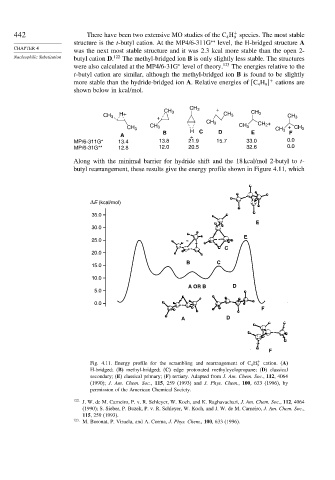
Along with the minimal barrier for hydride shift and the 18 kcal/mol 2-butyl to t-
butyl rearrangement, these results give the energy profile shown in Figure 4.11, which
ΔE (kcal/mol)
35.0
E
30.0
E
25.0
C
20.0
B C
15.0
10.0
A OR B D
5.0
0.0
F
A D
F
Fig. 4.11. Energy profile for the scrambling and rearrangement of C 4 H + cation. (A)
9
H-bridged; (B) methyl-bridged; (C) edge protonated methylcyclopropane; (D) classical
secondary; (E) classical primary; (F) tertiary. Adapted from J. Am. Chem. Soc., 112, 4064
(1990); J. Am. Chem. Soc., 115, 259 (1993) and J. Phys. Chem., 100, 633 (1996), by
permission of the American Chemical Society.
122
J. W. de M. Carneiro, P. v. R. Schleyer, W. Koch, and K. Raghavachari, J. Am. Chem. Soc., 112, 4064
(1990); S. Sieber, P. Buzek, P. v. R. Schleyer, W. Koch, and J. W. de M. Carneiro, J. Am. Chem. Soc.,
115, 259 (1993).
123 M. Boronat, P. Viruela, and A. Corma, J. Phys. Chem., 100, 633 (1996).