Page 464 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 464
445
D
18.5 SECTION 4.4
B B Structure and Reactions
(±120°) (0°) of Carbocation
B
B 15.2 15.6 (±60°) Intermediates
(±180°) 13.9
13.6
E
12.4
C
C cis-
trans- 10.6
9.6
A
0.00
+ H +
+ + Me H
Me 2 C CH 2 Me Me 2 CH CH Me + H
Me HC CH Me
Me Me Me Me
A B C D E
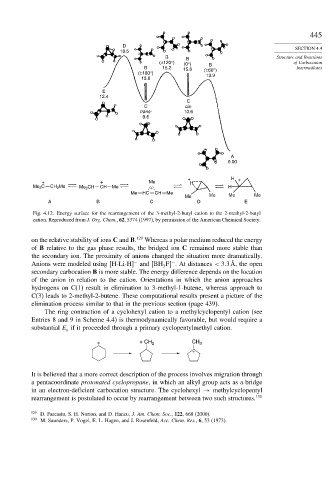
Fig. 4.12. Energy surface for the rearrangement of the 3-methyl-2-butyl cation to the 2-methyl-2-butyl
cation. Reproduced from J. Org. Chem., 62, 5374 (1997), by permission of the American Chemical Society.
on the relative stability of ions C and B. 129 Whereas a polar medium reduced the energy
of B relative to the gas phase results, the bridged ion C remained more stable than
the secondary ion. The proximity of anions changed the situation more dramatically.
−
Anions were modeled using H-Li-H and BH F . At distances < 3 3Å, the open
−
3
secondary carbocation B is more stable. The energy difference depends on the location
of the anion in relation to the cation. Orientations in which the anion approaches
hydrogens on C(1) result in elimination to 3-methyl-1-butene, whereas approach to
C(3) leads to 2-methyl-2-butene. These computational results present a picture of the
elimination process similar to that in the previous section (page 439).
The ring contraction of a cyclohexyl cation to a methylcyclopentyl cation (see
Entries 8 and 9 in Scheme 4.4) is thermodynamically favorable, but would require a
substantial E if it proceeded through a primary cyclopentylmethyl cation.
a
+ + CH 2 CH 3
+
It is believed that a more correct description of the process involves migration through
a pentacoordinate protonated cyclopropane, in which an alkyl group acts as a bridge
in an electron-deficient carbocation structure. The cyclohexyl → methylcyclopentyl
rearrangement is postulated to occur by rearrangement between two such structures. 130
129 D. Farcasiu, S. H. Norton, and D. Hancu, J. Am. Chem. Soc., 122, 668 (2000).
130
M. Saunders, P. Vogel, E. L. Hagen, and J. Rosenfeld, Acc. Chem. Res., 6, 53 (1973).