Page 459 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 459
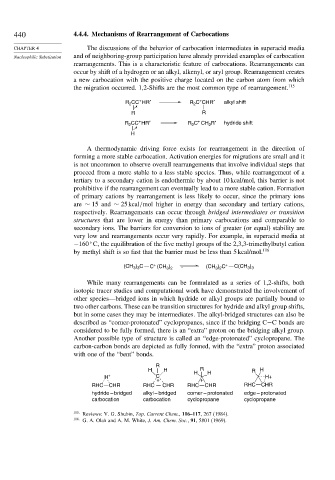
440 4.4.4. Mechanisms of Rearrangement of Carbocations
CHAPTER 4 The discussions of the behavior of carbocation intermediates in superacid media
Nucleophilic Substitution and of neighboring-group participation have already provided examples of carbocation
rearrangements. This is a characteristic feature of carbocations. Rearrangements can
occur by shift of a hydrogen or an alkyl, alkenyl, or aryl group. Rearrangement creates
a new carbocation with the positive charge located on the carbon atom from which
the migration occurred. 1,2-Shifts are the most common type of rearrangement. 115
CC HR′ R C CHR′ alkyl shift
+
+
R 2 2
R R
R CC HR′ R C CH R′ hydride shift
+
+
2
2
2
H
A thermodynamic driving force exists for rearrangement in the direction of
forming a more stable carbocation. Activation energies for migrations are small and it
is not uncommon to observe overall rearrangements that involve individual steps that
proceed from a more stable to a less stable species. Thus, while rearrangement of a
tertiary to a secondary cation is endothermic by about 10 kcal/mol, this barrier is not
prohibitive if the rearrangement can eventually lead to a more stable cation. Formation
of primary cations by rearrangement is less likely to occur, since the primary ions
are ∼ 15 and ∼ 25kcal/mol higher in energy than secondary and tertiary cations,
respectively. Rearrangements can occur through bridged intermediates or transition
structures that are lower in energy than primary carbocations and comparable to
secondary ions. The barriers for conversion to ions of greater (or equal) stability are
very low and rearrangements occur very rapidly. For example, in superacid media at
−160 C, the equilibration of the five methyl groups of the 2,3,3-trimethylbutyl cation
by methyl shift is so fast that the barrier must be less than 5 kcal/mol. 116
(CH ) C C (CH ) (CH ) C + C(CH )
+
3 3
3 3
3 2
3 2
While many rearrangements can be formulated as a series of 1,2-shifts, both
isotopic tracer studies and computational work have demonstrated the involvement of
other species—bridged ions in which hydride or alkyl groups are partially bound to
two other carbons. These can be transition structures for hydride and alkyl group shifts,
but in some cases they may be intermediates. The alkyl-bridged structures can also be
described as “corner-protonated” cyclopropanes, since if the bridging C−C bonds are
considered to be fully formed, there is an “extra” proton on the bridging alkyl group.
Another possible type of structure is called an “edge-protonated” cyclopropane. The
carbon-carbon bonds are depicted as fully formed, with the “extra” proton associated
with one of the “bent” bonds.
R
H H H R H R H
H + C H+
+ +
RHC CHR RHC CHR RHC CHR RHC CHR
hydride – bridged alkyl – bridged corner – protonated edge – protonated
carbocation carbocation cyclopropane cyclopropane
115 Reviews: V. G. Shubin, Top. Current Chem., 116–117, 267 (1984).
116
G. A. Olah and A. M. White, J. Am. Chem. Soc., 91, 5801 (1969).