Page 467 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 467
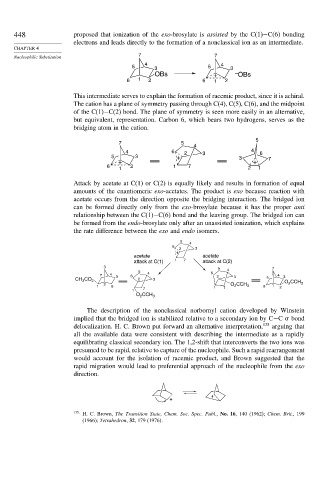
448 proposed that ionization of the exo-brosylate is assisted by the C(1)−C(6) bonding
electrons and leads directly to the formation of a nonclassical ion as an intermediate.
CHAPTER 4
7 7
Nucleophilic Substitution
4 4
5 3 5 3
OBs + – OBs
6 1 2 6 1 2
This intermediate serves to explain the formation of racemic product, since it is achiral.
The cation has a plane of symmetry passing through C(4), C(5), C(6), and the midpoint
of the C(1)−C(2) bond. The plane of symmetry is seen more easily in an alternative,
but equivalent, representation. Carbon 6, which bears two hydrogens, serves as the
bridging atom in the cation.
5
7 5
4
4 6 2 3 4 6
5 3 + 3 7
+ +
6 2 1 7
1 2 1
Attack by acetate at C(1) or C(2) is equally likely and results in formation of equal
amounts of the enantiomeric exo-acetates. The product is exo because reaction with
acetate occurs from the direction opposite the bridging interaction. The bridged ion
can be formed directly only from the exo-brosylate because it has the proper anti
relationship between the C(1)−C(6) bond and the leaving group. The bridged ion can
be formed from the endo-brosylate only after an unassisted ionization, which explains
the rate difference between the exo and endo isomers.
5
4
6
2 3
+
acetate acetate
attack at C(1) 1 7 attack at C(2)
3 5 7
5 6 4
7 4 5 6 4 2 3 5 4 3
CH 3 CO 2 2 3
O 2 CCH 3
1 2 6 1 7 O 2 CCH 3 6 1 2
1 7
O 2 CCH 3
The description of the nonclassical norbornyl cation developed by Winstein
implied that the bridged ion is stabilized relative to a secondary ion by C−C bond
delocalization. H. C. Brown put forward an alternative interpretation, 135 arguing that
all the available data were consistent with describing the intermediate as a rapidly
equilibrating classical secondary ion. The 1,2-shift that interconverts the two ions was
presumed to be rapid, relative to capture of the nucleophile. Such a rapid rearrangement
would account for the isolation of racemic product, and Brown suggested that the
rapid migration would lead to preferential approach of the nucleophile from the exo
direction.
+
+
135
H. C. Brown, The Transition State, Chem. Soc. Spec. Publ., No. 16, 140 (1962); Chem. Brit., 199
(1966); Tetrahedron, 32, 179 (1976).