Page 465 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 465
446 An E of 7 4±1kcal/mol for the rearrangement has been measured in the gas phase,
a
which is consistent with the protonated cyclopropane mechanism. 131
CHAPTER 4
Nucleophilic Substitution H
H H H H H H
+ H H H CH
+ 3 CH
+ H + 3
H H + H
H H H
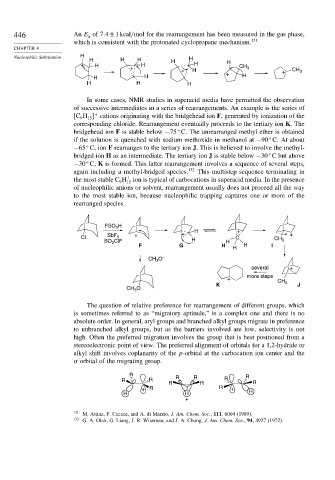
In some cases, NMR studies in superacid media have permitted the observation
of successive intermediates in a series of rearrangements. An example is the series of
C H cations originating with the bridgehead ion F, generated by ionization of the
+
9
15
corresponding chloride. Rearrangement eventually proceeds to the tertiary ion K. The
bridgehead ion F is stable below −75 C. The unrearranged methyl ether is obtained
if the solution is quenched with sodium methoxide in methanol at −90 C. At about
−65 C, ion F rearranges to the tertiary ion J. This is believed to involve the methyl-
bridged ion H as an intermediate. The tertiary ion J is stable below −30 C but above
−30 C, K is formed. This latter rearrangement involves a sequence of several steps,
again including a methyl-bridged species. 132 This multistep sequence terminating in
+
the most stable C H ion is typical of carbocations in superacid media. In the presence
9
15
of nucleophilic anions or solvent, rearrangement usually does not proceed all the way
to the most stable ion, because nucleophilic trapping captures one or more of the
rearranged species.
FSO H
3
H +
+
Cl SbF 5 + C CH +
SO ClF H H 3
2
F G H H H I
CH O –
3
several +
more steps
+ CH
K 3 J
CH O
3
The question of relative preference for rearrangement of different groups, which
is sometimes referred to as “migratory aptitude,” is a complex one and there is no
absolute order. In general, aryl groups and branched alkyl groups migrate in preference
to unbranched alkyl groups, but as the barriers involved are low, selectivity is not
high. Often the preferred migration involves the group that is best positioned from a
stereoelectronic point of view. The preferred alignment of orbitals for a 1,2-hydride or
alkyl shift involves coplanarity of the p-orbital at the carbocation ion center and the
orbital of the migrating group.
R R
R R R R R
R R R
+ R R +
H H H
+
131 M. Attina, F. Cacace, and A. di Marzio, J. Am. Chem. Soc., 111, 6004 (1989).
132
G. A. Olah, G. Liang, J. R. Wiseman, and J. A. Chong, J. Am. Chem. Soc., 94, 4927 (1972).