Page 452 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 452
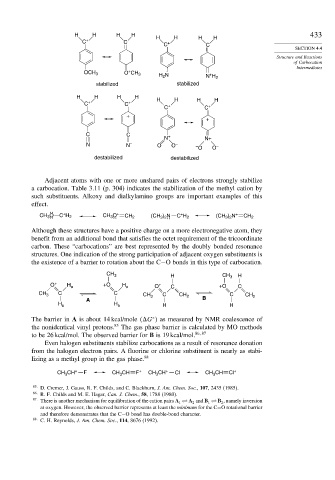
H H H H H H H H 433
C + C +
C C
SECTION 4.4
Structure and Reactions
of Carbocation
Intermediates
+
OCH 3 O CH 3 H 2 N +
N H 2
stabilized stabilized
H H H H H H H H
C + C + +
C C +
+
+
C C +
N N+
N N – O O – – –
O O
destabilized destabilized
Adjacent atoms with one or more unshared pairs of electrons strongly stabilize
a carbocation. Table 3.11 (p. 304) indicates the stabilization of the methyl cation by
such substituents. Alkoxy and dialkylamino groups are important examples of this
effect.
+
CH 3 O C H 2 CH 3 O + CH 2 (CH 3 ) 2 N C + H 2 (CH 3 ) 2 N + CH 2
Although these structures have a positive charge on a more electronegative atom, they
benefit from an additional bond that satisfies the octet requirement of the tricoordinate
carbon. These “carbocations” are best represented by the doubly bonded resonance
structures. One indication of the strong participation of adjacent oxygen substituents is
the existence of a barrier to rotation about the C−O bonds in this type of carbocation.
CH 3 H CH 3 H
O + H a +O H a O + C +O C
C C
CH 3 CH 3 C CH 2 C CH 2
A B
H b H b H H
The barrier in A is about 14 kcal/mole G as measured by NMR coalescence of
∗
the nonidentical vinyl protons. 85 The gas phase barrier is calculated by MO methods
to be 26 kcal/mol. The observed barrier for B is 19 kcal/mol. 86 87
Even halogen substituents stabilize carbocations as a result of resonance donation
from the halogen electron pairs. A fluorine or chlorine substituent is nearly as stabi-
lizing as a methyl group in the gas phase. 88
CH CH + F CH CH F + CH 3 CH + Cl CH 3 CH Cl +
3
3
85
D. Cremer, J. Gauss, R. F. Childs, and C. Blackburn, J. Am. Chem. Soc., 107, 2435 (1985).
86
R. F. Childs and M. E. Hagar, Can. J. Chem., 58, 1788 (1980).
87 There is another mechanism for equilibration of the cation pairs A 1 A 2 and B 1 B 2 , namely inversion
at oxygen. However, the observed barrier represents at least the minimum for the C=O rotational barrier
and therefore demonstrates that the C−O bond has double-bond character.
88
C. H. Reynolds, J. Am. Chem. Soc., 114, 8676 (1992).