Page 449 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 449
430
CHAPTER 4
1 104A
Nucleophilic Substitution
1 457A
1085A 1 459A
1 086A
1 103A
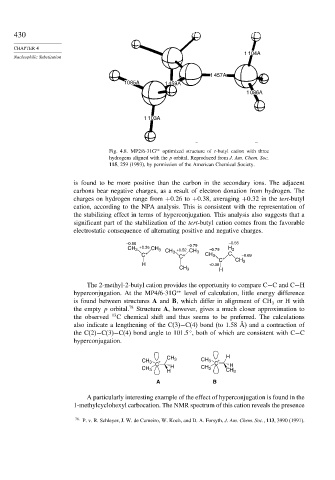
Fig. 4.8. MP2/6-31G ∗∗ optimized structure of t-butyl cation with three
hydrogens aligned with the p orbital. Reproduced from J. Am. Chem. Soc.
115, 259 (1993), by permission of the American Chemical Society.
is found to be more positive than the carbon in the secondary ions. The adjacent
carbons bear negative charges, as a result of electron donation from hydrogen. The
charges on hydrogen range from +0 26 to +0 38, averaging +0 32 in the tert-butyl
cation, according to the NPA analysis. This is consistent with the representation of
the stabilizing effect in terms of hyperconjugation. This analysis also suggests that a
significant part of the stabilization of the tert-butyl cation comes from the favorable
electrostatic consequence of alternating positive and negative charges.
–0.80 –0.79 –0.55
+0.35
CH 3 CH 3 CH 3 +0.52 CH 3 –0.79 H 2
C + C + CH 3 C –0.69
C + CH 3
H +0.30
CH 3 H
The 2-methyl-2-butyl cation provides the opportunity to compare C−C and C−H
hyperconjugation. At the MP4/6-31G ∗∗ level of calculation, little energy difference
is found between structures A and B, which differ in alignment of CH or H with
3
the empty p orbital. 78 Structure A, however, gives a much closer approximation to
the observed 13 C chemical shift and thus seems to be preferred. The calculations
also indicate a lengthening of the C(3)−C(4) bond (to 1.58 Å) and a contraction of
the C(2)−C(3)−C(4) bond angle to 101 5 , both of which are consistent with C−C
hyperconjugation.
H
CH 3 +
CH 3 + CH 3
C H C H
CH 3 CH 3
H CH 3
A B
A particularly interesting example of the effect of hyperconjugation is found in the
1-methylcyclohexyl carbocation. The NMR spectrum of this cation reveals the presence
78
P. v. R. Schleyer, J. W. de Carneiro, W. Koch, and D. A. Forsyth, J. Am. Chem. Soc., 113, 3990 (1991).