Page 498 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 498
X 479
H
SECTION 5.1
H
Addition of Hydrogen
Halides to Alkenes
X
H X
A change in the stereoselectivity is observed when the double bond is conju-
gated with a group that can stabilize a carbocation intermediate. Most of the specific
cases involve an aryl substituent. Examples of alkenes that give primarily syn
addition are Z- and E-1-phenylpropene, 11 cis- and trans-ß-t-butylstyrene, 12 1-phenyl-
4-t-butylcyclohexene, 13 and indene. 14 The mechanism proposed for these reactions
features an ion pair as the key intermediate. Owing to the greater stability of the
benzylic carbocations formed in these reactions, concerted attack by halide ion is not
required for protonation. If the ion pair formed by alkene protonation collapses to
product faster than rotation takes place, syn addition occurs because the proton and
halide ion are initially on the same face of the molecule.
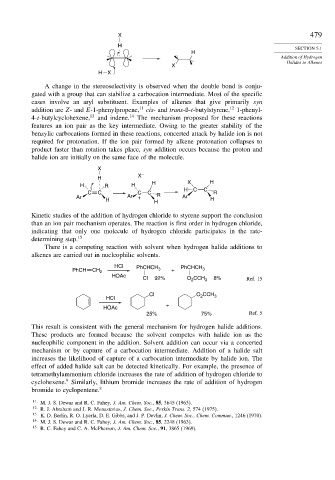
X
X –
H
H R H H X H
H C C
C C C C R
Ar Ar + R Ar
H H H
Kinetic studies of the addition of hydrogen chloride to styrene support the conclusion
than an ion pair mechanism operates. The reaction is first order in hydrogen chloride,
indicating that only one molecule of hydrogen chloride participates in the rate-
determining step. 15
There is a competing reaction with solvent when hydrogen halide additions to
alkenes are carried out in nucleophilic solvents.
HCl PhCHCH PhCHCH
PhCH CH 2 3 + 3
HOAc
Cl 92% O CCH 3 8% Ref. 15
2
Cl O CCH
HCl 2 3
HOAc +
25% 75% Ref. 5
This result is consistent with the general mechanism for hydrogen halide additions.
These products are formed because the solvent competes with halide ion as the
nucleophilic component in the addition. Solvent addition can occur via a concerted
mechanism or by capture of a carbocation intermediate. Addition of a halide salt
increases the likelihood of capture of a carbocation intermediate by halide ion. The
effect of added halide salt can be detected kinetically. For example, the presence of
tetramethylammonium chloride increases the rate of addition of hydrogen chloride to
9
cyclohexene. Similarly, lithium bromide increases the rate of addition of hydrogen
bromide to cyclopentene. 8
11 M. J. S. Dewar and R. C. Fahey, J. Am. Chem. Soc., 85, 3645 (1963).
12
R. J. Abraham and J. R. Monasterios, J. Chem. Soc., Perkin Trans. 2, 574 (1975).
13 K. D. Berlin, R. O. Lyerla, D. E. Gibbs, and J. P. Devlin, J. Chem. Soc., Chem. Commun., 1246 (1970).
14 M. J. S. Dewar and R. C. Fahey, J. Am. Chem. Soc., 85, 2248 (1963).
15
R. C. Fahey and C. A. McPherson, J. Am. Chem. Soc., 91, 3865 (1969).