Page 494 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 494
475
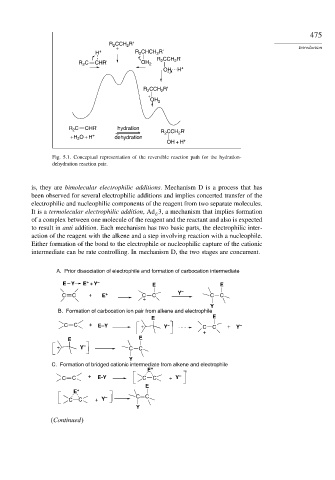
R CCH R' Introduction
2
2
H + + R CHCH R'
2
2
+ R CCH R'
2
2
R C CHR' OH 2
2
OH H +
CCH R'
R 2 2
+
OH 2
R C CHR' hydration R CCH R'
2
+
+H O + H dehydration 2 2
2
OH + H +
Fig. 5.1. Conceptual representation of the reversible reaction path for the hydration-
dehydration reaction pair.
is, they are bimolecular electrophilic additions. Mechanism D is a process that has
been observed for several electrophilic additions and implies concerted transfer of the
electrophilic and nucleophilic components of the reagent from two separate molecules.
It is a termolecular electrophilic addition,Ad 3, a mechanism that implies formation
E
of a complex between one molecule of the reagent and the reactant and also is expected
to result in anti addition. Each mechanism has two basic parts, the electrophilic inter-
action of the reagent with the alkene and a step involving reaction with a nucleophile.
Either formation of the bond to the electrophile or nucleophilic capture of the cationic
intermediate can be rate controlling. In mechanism D, the two stages are concurrent.
A. Prior dissociation of electrophile and formation of carbocation intermediate
+
–
E – Y E + Y E E
C C + E + C C Y – C C
+
Y
B. Formation of carbocation ion pair from alkene and electrophile
E E
C C + E–Y + Y – C C + Y –
+
E E
+ Y – C C
Y
C. Formation of bridged cationic intermediate from alkene and electrophile
E +
C C + E-Y C C + Y –
E
E +
C C + Y – C C
Y
Continued