Page 499 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 499
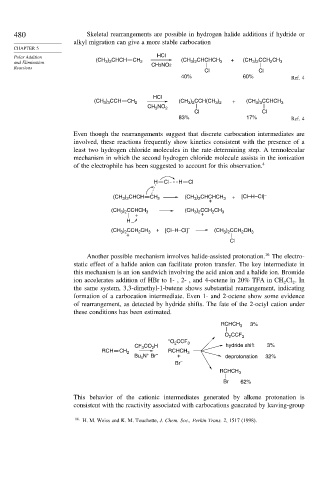
480 Skeletal rearrangements are possible in hydrogen halide additions if hydride or
alkyl migration can give a more stable carbocation
CHAPTER 5
Polar Addition HCl
3 2
3 2
2
3 2
and Elimination (CH ) CHCH CH 2 (CH ) CHCHCH 3 + (CH ) CCH CH 3
Reactions CH3NO2
Cl Cl
40% 60% Ref. 4
HCl
(CH ) CCH CH 2 (CH ) CCH(CH ) + (CH ) CCHCH 3
3 2
3 2
3 3
3 3
NO
CH 3 2
Cl Cl
83% 17% Ref. 4
Even though the rearrangements suggest that discrete carbocation intermediates are
involved, these reactions frequently show kinetics consistent with the presence of a
least two hydrogen chloride molecules in the rate-determining step. A termolecular
mechanism in which the second hydrogen chloride molecule assists in the ionization
of the electrophile has been suggested to account for this observation. 4
H Cl H Cl
(CH ) CHCH CH 2 (CH ) CHCHCH 3 + [Cl–H–Cl] –
3 2
3 2
+
(CH ) CCHCH 3 (CH ) CCH CH 3
2
3 2
3 2
+ +
H
(CH ) CCH CH 3 + [Cl–H–Cl] – (CH ) CCH CH 3
2
3 2
2
3 2
+
Cl
Another possible mechanism involves halide-assisted protonation. 16 The electro-
static effect of a halide anion can facilitate proton transfer. The key intermediate in
this mechanism is an ion sandwich involving the acid anion and a halide ion. Bromide
ion accelerates addition of HBr to 1- , 2- , and 4-octene in 20% TFA in CH Cl .In
2 2
the same system, 3,3-dimethyl-1-butene shows substantial rearrangement, indicating
formation of a carbocation intermediate. Even 1- and 2-octene show some evidence
of rearrangement, as detected by hydride shifts. The fate of the 2-octyl cation under
these conditions has been estimated.
RCHCH 3 3%
O CCF 3
2
– O CCF
CF CO H 2 3 hydride shift 3%
3
2
RCH CH 2 RCHCH 3
+
Bu N Br – + deprotonation 32%
4
Br –
RCHCH 3
Br 62%
This behavior of the cationic intermediates generated by alkene protonation is
consistent with the reactivity associated with carbocations generated by leaving-group
16
H. M. Weiss and K. M. Touchette, J. Chem. Soc., Perkin Trans. 2, 1517 (1998).