Page 503 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 503
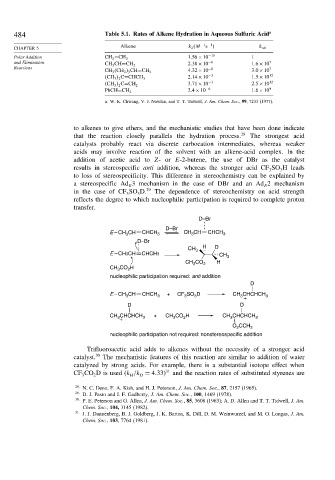
484 Table 5.1. Rates of Alkene Hydration in Aqueous Sulfuric Acid a
s
Alkene k 2 M −1 −1
CHAPTER 5 k rel
Polar Addition CH 2 =CH 2 1 56×10 −15 1
and Elimination 2 38×10 −8 1 6×10 7
CH 3 CH=CH 2
Reactions −8 7
CH 3 CH 2 3 CH=CH 2 4 32×10 3 0×10
2 14×10 −3 1 5×10 12
CH 3 2 C=CHCH 3
3 71×10 −3 2 5×10 12
CH 3 2 C=CH 2
2 4×10 −6 1 6×10 9
PhCH=CH 2
a. W. K. Chwang, V. J. Nowlan, and T. T. Tidwell, J. Am. Chem. Soc., 99, 7233 (1977).
to alkenes to give ethers, and the mechanistic studies that have been done indicate
that the reaction closely parallels the hydration process. 28 The strongest acid
catalysts probably react via discrete carbocation intermediates, whereas weaker
acids may involve reaction of the solvent with an alkene-acid complex. In the
addition of acetic acid to Z-or E-2-butene, the use of DBr as the catalyst
results in stereospecific anti addition, whereas the stronger acid CF SO H leads
3
3
to loss of stereospecificity. This difference in stereochemistry can be explained by
a stereospecific Ad 3 mechanism in the case of DBr and an Ad 2 mechanism
E E
in the case of CF SO D. 29 The dependence of stereochemistry on acid strength
3 3
reflects the degree to which nucleophilic participation is required to complete proton
transfer.
D–Br
D–Br
E – CH CH CHCH 3 CH CH CHCH 3
3
3
D–Br
H D
CH 3
E – CH3CH CHCH3 CH 3
CH CO 2 H
3
CH CO H
2
3
nucleophilic participation required: anti addition
D
E – CH CH CHCH 3 + CF SO D CH CHCHCH 3
3
3
3
3
+
D D
CH CHCHCH + CH CO H CH CHCHCH
3
+ 3 3 2 3 3
O 2 CCH 3
nucleophilic participation not required: nonstereospecific addition
Trifluoroacetic acid adds to alkenes without the necessity of a stronger acid
catalyst. 30 The mechanistic features of this reaction are similar to addition of water
catalyzed by strong acids. For example, there is a substantial isotope effect when
CF CO D is used (k /k = 4 33) 31 and the reaction rates of substituted styrenes are
3
D
2
H
28 N. C. Deno, F. A. Kish, and H. J. Peterson, J. Am. Chem. Soc., 87, 2157 (1965).
29
D. J. Pasto and J. F. Gadberry, J. Am. Chem. Soc., 100, 1469 (1978).
30 P. E. Peterson and G. Allen, J. Am. Chem. Soc., 85, 3608 (1963); A. D. Allen and T. T. Tidwell, J. Am.
Chem. Soc., 104, 3145 (1982).
31
J. J. Dannenberg, B. J. Goldberg, J. K. Barton, K. Dill, D. M. Weinwurzel, and M. O. Longas, J. Am.
Chem. Soc., 103, 7764 (1981).