Page 506 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 506
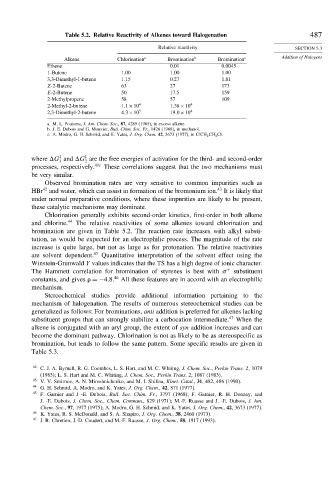
Table 5.2. Relative Reactivity of Alkenes toward Halogenation 487
Relative reactivity SECTION 5.3
Alkene Chlorination a Bromination b Bromination c Addition of Halogens
Ethene 0.01 0.0045
1-Butene 1.00 1.00 1.00
3,3-Dimethyl-1-butene 1.15 0.27 1.81
Z-2-Butene 63 27 173
E-2-Butene 50 17.5 159
2-Methylpropene 58 57 109
2-Methyl-2-butene 1 1×10 4 1 38×10 4
2,3-Dimethyl-2-butene 4 3×10 5 19 0×10 4
a. M. L. Poutsma, J. Am. Chem. Soc., 87, 4285 (1965), in excess alkene.
b. J. E. Dubois and G. Mouvier, Bull. Chim. Soc. Fr., 1426 (1968), in methanol.
c. A. Modro, G. H. Schmid, and K. Yates, J. Org. Chem. 42, 3673 (1977), in ClCH 2 CH 2 Cl.
‡
‡
where G and G are the free energies of activation for the third- and second-order
3 2
processes, respectively. 41c These correlations suggest that the two mechanisms must
be very similar.
Observed bromination rates are very sensitive to common impurities such as
43
42
HBr and water, which can assist in formation of the bromonium ion. It is likely that
under normal preparative conditions, where these impurities are likely to be present,
these catalytic mechanisms may dominate.
Chlorination generally exhibits second-order kinetics, first-order in both alkene
and chlorine. 44 The relative reactivities of some alkenes toward chlorination and
bromination are given in Table 5.2. The reaction rate increases with alkyl substi-
tution, as would be expected for an electrophilic process. The magnitude of the rate
increase is quite large, but not as large as for protonation. The relative reactivities
are solvent dependent. 45 Quantitative interpretation of the solvent effect using the
Winstein-Grunwald Y values indicates that the TS has a high degree of ionic character.
The Hammett correlation for bromination of styrenes is best with
+ substituent
constants, and gives
=−4 8. 46 All these features are in accord with an electrophilic
mechanism.
Stereochemical studies provide additional information pertaining to the
mechanism of halogenation. The results of numerous stereochemical studies can be
generalized as follows: For brominations, anti addition is preferred for alkenes lacking
substituent groups that can strongly stabilize a carbocation intermediate. 47 When the
alkene is conjugated with an aryl group, the extent of syn addition increases and can
become the dominant pathway. Chlorination is not as likely to be as stereospecific as
bromination, but tends to follow the same pattern. Some specific results are given in
Table 5.3.
42
C. J. A. Byrnell, R. G. Coombes, L. S. Hart, and M. C. Whiting, J. Chem. Soc., Perkin Trans. 2 1079
(1983); L. S. Hart and M. C. Whiting, J. Chem. Soc., Perkin Trans. 2, 1087 (1983).
43 V. V. Smirnov, A. N. Miroshnichenko, and M. I. Shilina, Kinet. Catal., 31, 482, 486 (1990).
44
G. H. Schmid, A. Modro, and K. Yates, J. Org. Chem., 42, 871 (1977).
45
F. Garnier and J -E. Dubois, Bull. Soc. Chim. Fr., 3797 (1968); F. Garnier, R. H. Donnay, and
J. -E. Dubois, J. Chem. Soc., Chem. Commun., 829 (1971); M.-F. Ruasse and J. -E. Dubois, J. Am.
Chem. Soc., 97, 1977 (1975); A. Modro, G. H. Schmid, and K. Yates, J. Org. Chem., 42, 3673 (1977).
46 K. Yates, R. S. McDonald, and S. A. Shapiro, J. Org. Chem., 38, 2460 (1973).
47
J. R. Chretien, J.-D. Coudert, and M.-F. Ruasse, J. Org. Chem., 58, 1917 (1993).