Page 508 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 508
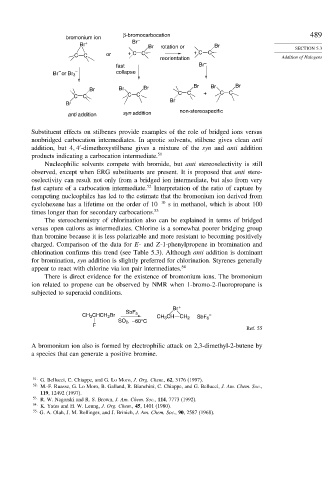
β-bromocarbocation 489
bromonium ion –
Br + Br
Br rotation or Br SECTION 5.3
C C or + CC + C C Addition of Halogens
reorientation
fast Br –
– – collapse
Br or Br 3
Br Br Br Br Br Br
CC CC C C + C C
Br
Br
anti addition syn addition non-stereospecific
Substituent effects on stilbenes provide examples of the role of bridged ions versus
nonbridged carbocation intermediates. In aprotic solvents, stilbene gives clean anti
addition, but 4 4 -dimethoxystilbene gives a mixture of the syn and anti addition
products indicating a carbocation intermediate. 51
Nucleophilic solvents compete with bromide, but anti stereoselectivity is still
observed, except when ERG substituents are present. It is proposed that anti stere-
oselectivity can result not only from a bridged ion intermediate, but also from very
fast capture of a carbocation intermediate. 52 Interpretation of the ratio of capture by
competing nucleophiles has led to the estimate that the bromonium ion derived from
cyclohexene has a lifetime on the order of 10 −10 s in methanol, which is about 100
times longer than for secondary carbocations. 53
The stereochemistry of chlorination also can be explained in terms of bridged
versus open cations as intermediates. Chlorine is a somewhat poorer bridging group
than bromine because it is less polarizable and more resistant to becoming positively
charged. Comparison of the data for E- and Z-1-phenylpropene in bromination and
chlorination confirms this trend (see Table 5.3). Although anti addition is dominant
for bromination, syn addition is slightly preferred for chlorination. Styrenes generally
appear to react with chlorine via ion pair intermediates. 54
There is direct evidence for the existence of bromonium ions. The bromonium
ion related to propene can be observed by NMR when 1-bromo-2-fluoropropane is
subjected to superacid conditions.
Br +
SbF 5
CH 3 CHCH 2 Br CH 3 CH CH 2 –
SO 2 , –60°C SbF 6
F
Ref. 55
A bromonium ion also is formed by electrophilic attack on 2,3-dimethyl-2-butene by
a species that can generate a positive bromine.
51
G. Bellucci, C. Chiappe, and G. Lo Moro, J. Org. Chem., 62, 3176 (1997).
52
M.-F. Ruasse, G. Lo Moro, B. Galland, R. Bianchini, C. Chiappe, and G. Bellucci, J. Am. Chem. Soc.,
119, 12492 (1997).
53 R. W. Nagorski and R. S. Brown, J. Am. Chem. Soc., 114, 7773 (1992).
54 K. Yates and H. W. Leung, J. Org. Chem., 45, 1401 (1980).
55
G. A. Olah, J. M. Bollinger, and J. Brinich, J. Am. Chem. Soc., 90, 2587 (1968).