Page 509 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 509
490 Br +
+ –
–
(CH 3 ) 2 C C(CH ) + Br-C N Sb F 5 CH 3 CH 3 [C NSb 5 ]
CHAPTER 5 3 2 CH 3
CH 3
Polar Addition
and Elimination Ref. 56
Reactions
The highly hindered alkene adamantylideneadamantane forms a bromonium ion
that crystallizes as a tribromide salt. This particular bromonium ion does not react
further because of extreme steric hindrance to back-side approach by bromide ion. 57
Other very hindered alkenes allow observation of both the initial complex with Br
2
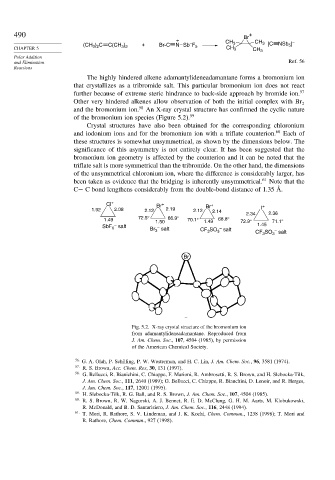
and the bromonium ion. 58 An X-ray crystal structure has confirmed the cyclic nature
of the bromonium ion species (Figure 5.2). 59
Crystal structures have also been obtained for the corresponding chloronium
and iodonium ions and for the bromonium ion with a triflate counterion. 60 Each of
these structures is somewhat unsymmetrical, as shown by the dimensions below. The
significance of this asymmetry is not entirely clear. It has been suggested that the
bromonium ion geometry is affected by the counterion and it can be noted that the
triflate salt is more symmetrical than the tribromide. On the other hand, the dimensions
of the unsymmetrical chloronium ion, where the difference is considerably larger, has
been taken as evidence that the bridging is inherently unsymmetrical. 61 Note that the
C− C bond lengthens considerably from the double-bond distance of 1.35 Å.
Cl + Br + Br + +
1.92 2.08 2.12 2.19 2.12 2.14 2.34 I 2.36
72.5° 66.9° 68.8°
1.49 1.50 70.1° 1.49 72.9° 71.1°
–
SbF salt Br salt CF SO salt 1.45
–
–
6
–
3
3
3
3
CF 3 SO salt
Br
Fig. 5.2. X-ray crystal structure of the bromonium ion
from adamantylideneadamantane. Reproduced from
J. Am. Chem. Soc., 107, 4504 (1985), by permission
of the American Chemical Society.
56 G. A. Olah, P. Schilling, P. W. Westerman, and H. C. Lin, J. Am. Chem. Soc., 96, 3581 (1974).
57 R. S. Brown, Acc. Chem. Res, 30, 131 (1997).
58
G. Bellucci, R. Bianichini, C. Chiappe, F. Marioni, R. Ambrosetti, R. S. Brown, and H. Slebocka-Tilk,
J. Am. Chem. Soc., 111, 2640 (1989); G. Bellucci, C. Chiappe, R. Bianchini, D. Lenoir, and R. Herges,
J. Am. Chem. Soc., 117, 12001 (1995).
59 H. Slebocka-Tilk, R. G. Ball, and R. S. Brown, J. Am. Chem. Soc., 107, 4504 (1985).
60 R. S. Brown, R. W. Nagorski, A. J. Bennet, R. E. D. McClung, G. H. M. Aarts, M. Klobukowski,
R. McDonald, and B. D. Santarisiero, J. Am. Chem. Soc., 116, 2448 (1994).
61
T. Mori, R. Rathore, S. V. Lindeman, and J. K. Kochi, Chem. Commun., 1238 (1998); T. Mori and
R. Rathore, Chem. Commun., 927 (1998).