Page 511 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 511
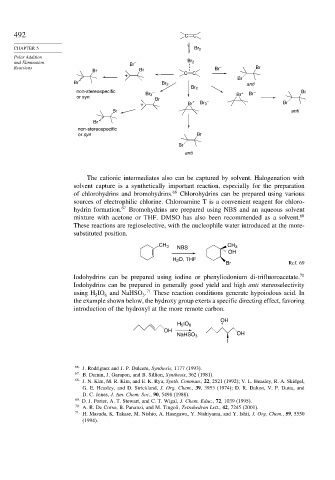
492 C C
CHAPTER 5 Br 2
Polar Addition
and Elimination Br – Br 2
Reactions Br – Br
Br Br
+ C C
Br
Br Br 2 anti
Br 2
non-stereospecific – + – Br
Br 3 Br Br
or syn
Br
+ Br + Br 3 – Br
Br anti
Br
non-stereospecific
or syn Br
Br
anti
The cationic intermediates also can be captured by solvent. Halogenation with
solvent capture is a synthetically important reaction, especially for the preparation
of chlorohydrins and bromohydrins. 66 Chlorohydrins can be prepared using various
sources of electrophilic chlorine. Chloroamine T is a convenient reagent for chloro-
hydrin formation. 67 Bromohydrins are prepared using NBS and an aqueous solvent
mixture with acetone or THF. DMSO has also been recommended as a solvent. 68
These reactions are regioselective, with the nucleophile water introduced at the more-
substituted position.
CH 3 CH 3
NBS
OH
H 2 O, THF
Br Ref. 69
Iodohydrins can be prepared using iodine or phenyliodonium di-trifluoroacetate. 70
Iodohydrins can be prepared in generally good yield and high anti stereoselectivity
using H IO and NaHSO . 71 These reaction conditions generate hypoiodous acid. In
5 6 3
the example shown below, the hydroxy group exerts a specific directing effect, favoring
introduction of the hydroxyl at the more remote carbon.
OH
H 5 IO 6
OH
NaHSO 3 OH
I
66
J. Rodriguez and J. P. Dulcere, Synthesis, 1177 (1993).
67 B. Damin, J. Garapon, and B. Sillion, Synthesis, 362 (1981).
68
J. N. Kim, M. R. Kim, and E. K. Ryu, Synth. Commun., 22, 2521 (1992); V. L. Heasley, R. A. Skidgel,
G. E. Heasley, and D. Strickland, J. Org. Chem., 39, 3953 (1974); D. R. Dalton, V. P. Dutta, and
D. C. Jones, J. Am. Chem. Soc., 90, 5498 (1988).
69
D. J. Porter, A. T. Stewart, and C. T. Wigal, J. Chem. Educ., 72, 1039 (1995).
70 A. R. De Corso, B. Panunzi, and M. Tingoli, Tetrahedron Lett., 42, 7245 (2001).
71
H. Masuda, K. Takase, M. Nishio, A. Hasegawa, Y. Nishiyama, and Y. Ishii, J. Org. Chem., 59, 5550
(1994).