Page 513 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 513
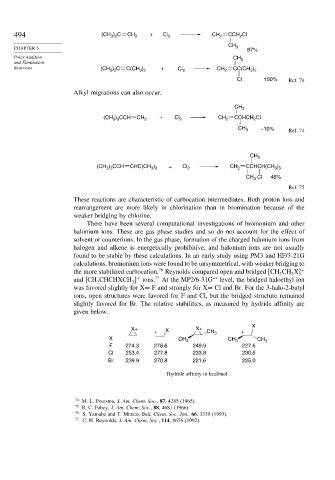
494 (CH ) C CH 2 + Cl 2 CH 2 CCH Cl
2
3 2
CH
CHAPTER 5 3 87%
Polar Addition CH 3
and Elimination
Reactions (CH ) C C(CH ) + Cl 2 CH 2 CC(CH )
3 2
3 2
3 2
Cl 100% Ref. 74
Alkyl migrations can also occur.
CH 3
(CH ) CCH CH 2 + Cl 2 CH 2 CCHCH Cl
2
3 3
CH 3 ~10% Ref. 74
CH 3
(CH ) CCH CHC(CH ) + Cl 2 CH 2 CCHCH(CH )
3 3
3 3
3 3
CH 3 Cl 46%
Ref. 75
These reactions are characteristic of carbocation intermediates. Both proton loss and
rearrangement are more likely in chlorination than in bromination because of the
weaker bridging by chlorine.
There have been several computational investigations of bromonium and other
halonium ions. These are gas phase studies and so do not account for the effect of
solvent or counterions. In the gas phase, formation of the charged halonium ions from
halogen and alkene is energetically prohibitive, and halonium ions are not usually
found to be stable by these calculations. In an early study using PM3 and HF/3-21G
calculations, bromonium ions were found to be unsymmetrical, with weaker bridging to
76
the more stabilized carbocation. Reynolds compared open and bridged [CH CH X +
2
2
+
and CH CHCHXCH ions. 77 At the MP2/6-31G ∗∗ level, the bridged haloethyl ion
3
3
was favored slightly for X= F and strongly for X= Cl and Br. For the 3-halo-2-butyl
ions, open structures were favored for F and Cl, but the bridged structure remained
slightly favored for Br. The relative stabilities, as measured by hydride affinity are
given below.
X
X+ X X+
+ CH 3 +
X CH 3 CH 3 CH 3
F 274.3 278.6 249.9 227.6
Cl 253.4 277.8 233.8 230.6
Br 239.9 270.8 221.6 225.0
Hydride affinity in kcal/mol
74
M. L. Poutsma, J. Am. Chem. Soc., 87, 4285 (1965).
75
R. C. Fahey, J. Am. Chem. Soc., 88, 4681 (1966).
76 S. Yamabe and T. Minato, Bull. Chem. Soc. Jpn., 66, 3339 (1993).
77
C. H. Reynolds, J. Am. Chem. Soc., 114, 8676 (1992).